Question: just the final answer Question 8 For the given reaction, the reaction quotient Q is calculated: A (g) +B (g) =C() At constant temperature, which
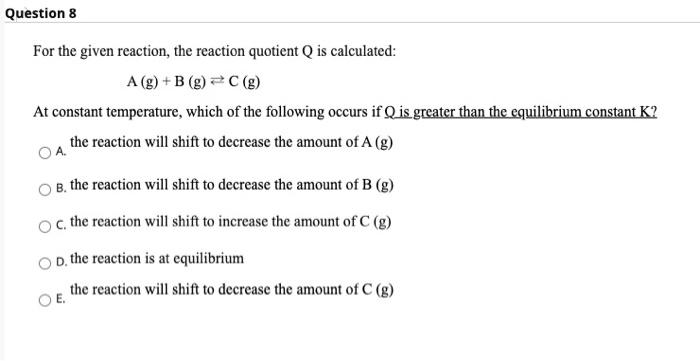
Question 8 For the given reaction, the reaction quotient Q is calculated: A (g) +B (g) =C() At constant temperature, which of the following occurs if Q is greater than the equilibrium constant K? the reaction will shift to decrease the amount of A (8) B. the reaction will shift to decrease the amount of B (g) c the reaction will shift to increase the amount of C (g) D. the reaction is at equilibrium the reaction will shift to decrease the amount of C (g)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


