A reaction is to be carried out in the packed-bed reactor shown in Figure P12-19C. PFR with
Question:
A reaction is to be carried out in the packed-bed reactor shown in Figure P12-19C.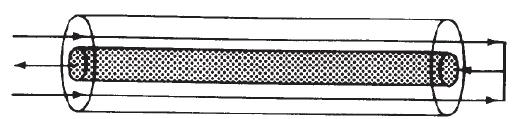
PFR with heat exchange. The reactants enter the annular space between an outer insulated tube and an inner tube containing the catalyst. No reaction takes place in the annular region. Heat transfer between the gas in this packed-bed reactor and the gas flowing counter currently in the annular space occurs along the length of the reactor. The overall heat transfer coefficient is 5 W/m2 · K. Plot the conversion and temperature as a function of reactor length for the data given in Problem P12-7B.
Data from Problem P12-7B.
Use the data in Problem P11-4A for the following reaction. The elementary, irreversible, organic liquid-phase reaction
A + B → C is carried out in a flow reactor. An equal molar feed in A and B enters at 27°C, and the volumetric flow rate is 2 dm3/s and CA0 = 0.1 kmol/m3.
Additional information:![]() (a) Calculate the conversion when the reaction is carried out adiabatically in one 500-dm3 CSTR and then compare the results with the two adiabatic 250-dm3 CSTRs in series.
(a) Calculate the conversion when the reaction is carried out adiabatically in one 500-dm3 CSTR and then compare the results with the two adiabatic 250-dm3 CSTRs in series.
The reversible reaction is now carried out in a PFR with a heat exchanger. Plot and then analyze X, Xe, T, Ta, Qr, Qg, and the rate, –rA, for
the following cases:
(b) Constant heat-exchanger temperature Ta
(c) Co-current heat exchanger Ta.
(d) Countercurrent heat exchanger Ta.
(e) Adiabatic operation
(f) Make a table comparing all your results (e.g., X, Xe, T, Ta). Write a paragraph describing what you find.
(g) Plot Qr and Ta as a function of V necessary to maintain isothermal operation.
Data From Problem P11-4A
The elementary, irreversible, organic liquid-phase reaction A + B → C is carried out adiabatically in a flow reactor. An equal molar feed in A and B enters at 27°C, and the volumetric flow rate is 2 dm3/s and CA0 = 0.1 kmol/m3.
Additional information:
PFR
a. Plot and then analyze the conversion and temperature as a function of PFR volume up to where X = 0.85. Describe the trends.
b. What is the maximum inlet temperature one could have so that the boiling point of the liquid (550 K) would not be exceeded even for complete conversion?
c. Plot the heat that must be removed along the reactor (Q˙ vs. V) to maintain isothermal operation.
d. Plot and then analyze the conversion and temperature profiles up to a PFR reactor volume of 10 dm3 for the case when the reaction is reversible with KC = 10 m3/kmol at 450 K. Plot the equilibrium conversion profile. How are the trends different than part (a)?
CSTR
e. What is the CSTR volume necessary to achieve 90% conversion?
BR
f. The reaction is next carried out in a 25 dm3 batch reactor charged with NA0 = 10 moles. Plot the number of moles of A, NA, the conversion, and the temperature as a function of time.
Step by Step Answer:





