Question: kbT = = kb = 1.3806 x 10-2 J/K; B = KT; 9(B) = ; 9; exp[-E;B] ; BB hlaj, Ej, a,B) = InW +
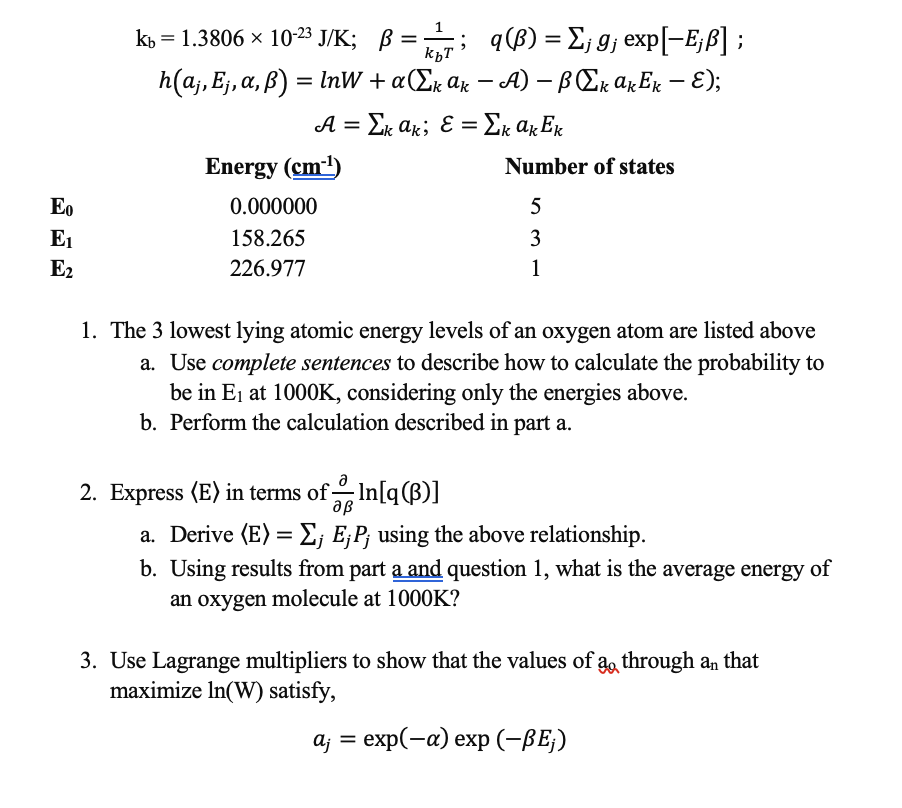
kbT = = kb = 1.3806 x 10-2 J/K; B = KT; 9(B) = ; 9; exp[-E;B] ; BB hlaj, Ej, a,B) = InW + a(Ek Ax A) B Ek akEx E); A = Ek ak; E = Ex ax Ex Energy (cm-1) = = Number of states 5 EO E1 E2 0.000000 158.265 226.977 3 1 1. The 3 lowest lying atomic energy levels of an oxygen atom are listed above a. Use complete sentences to describe how to calculate the probability to be in E at 1000K, considering only the energies above. b. Perform the calculation described in part a. ap = 2. Express (E) in terms of In[q(B)] a. Derive (E) = Ej EjP; using the above relationship. b. Using results from part a and question 1, what is the average energy of an oxygen molecule at 1000K? 3. Use Lagrange multipliers to show that the values of 2, through an that ao maximize In(W) satisfy, a; = exp(-a) exp (-BE;) kbT = = kb = 1.3806 x 10-2 J/K; B = KT; 9(B) = ; 9; exp[-E;B] ; BB hlaj, Ej, a,B) = InW + a(Ek Ax A) B Ek akEx E); A = Ek ak; E = Ex ax Ex Energy (cm-1) = = Number of states 5 EO E1 E2 0.000000 158.265 226.977 3 1 1. The 3 lowest lying atomic energy levels of an oxygen atom are listed above a. Use complete sentences to describe how to calculate the probability to be in E at 1000K, considering only the energies above. b. Perform the calculation described in part a. ap = 2. Express (E) in terms of In[q(B)] a. Derive (E) = Ej EjP; using the above relationship. b. Using results from part a and question 1, what is the average energy of an oxygen molecule at 1000K? 3. Use Lagrange multipliers to show that the values of 2, through an that ao maximize In(W) satisfy, a; = exp(-a) exp (-BE;)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


