Question: Kc=1.8 Express your answer using two significant figures. Enter your answers numerically separated by commas. View Available Hint(s) Kc=2.0102 Express your answer using two significant
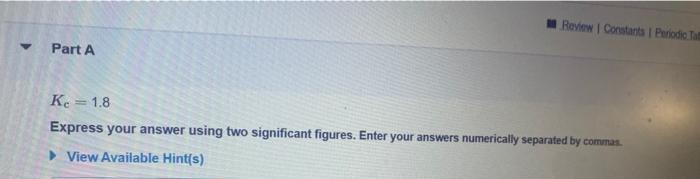
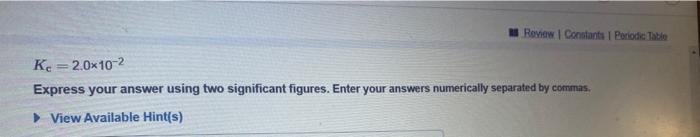

Kc=1.8 Express your answer using two significant figures. Enter your answers numerically separated by commas. View Available Hint(s) Kc=2.0102 Express your answer using two significant figures. Enter your answers numerically separated by commas. View Available Hint(s) Kc=1.8105 Express your answer using two significant figures. Enter your answers numerically separated by commas. View Available Hint(s)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


