Question: KCl has the NaCl type structure which is fcc with two-atom basis, one at (0,0,0) and the other at (1/2,1/2,1/2). Assume that the atomic
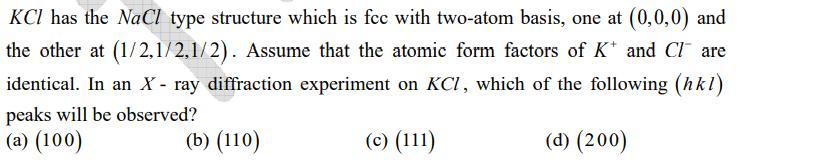
KCl has the NaCl type structure which is fcc with two-atom basis, one at (0,0,0) and the other at (1/2,1/2,1/2). Assume that the atomic form factors of K* and Clare identical. In an X-ray diffraction experiment on KCl, which of the following (hkl) peaks will be observed? (a) (100) (b) (110) (c) (111) (d) (200)
Step by Step Solution
3.46 Rating (153 Votes )
There are 3 Steps involved in it
The detailed ... View full answer

Get step-by-step solutions from verified subject matter experts


