Question: ken:0:07:26 Deniece Miranda: Attempt 1 Question 4 (3 points) Given (7.32x10^6) grams of glucose, C6H12O6, calculate the grams of carbon dioxide, CO formed upon
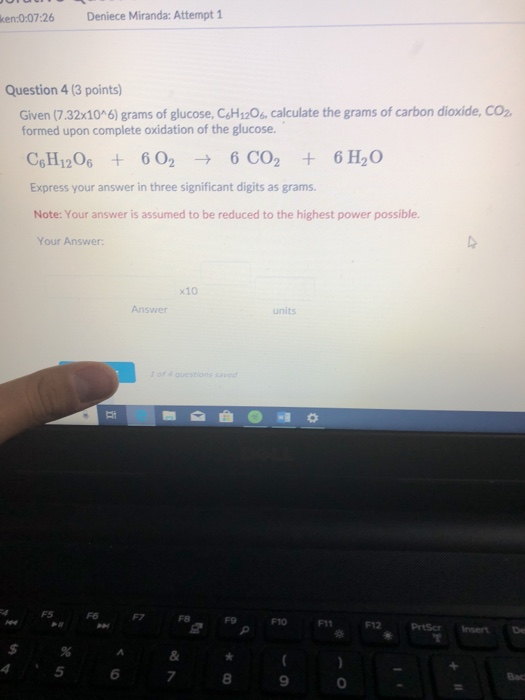
ken:0:07:26 Deniece Miranda: Attempt 1 Question 4 (3 points) Given (7.32x10^6) grams of glucose, C6H12O6, calculate the grams of carbon dioxide, CO formed upon complete oxidation of the glucose. C6H12O6 + 6 026 CO2 + 6HO Express your answer in three significant digits as grams. Note: Your answer is assumed to be reduced to the highest power possible. Your Answer: F5 F6 B P Answer F7 x10 1 of 4 questions saved FB & 7 di * 8 a units F10 S F11 * F12 PrtScr T De Bac
Step by Step Solution
3.30 Rating (144 Votes )
There are 3 Steps involved in it
SOLUTION Given reaction C6H12O6 602 given mass of glucose 732 106 g ... View full answer

Get step-by-step solutions from verified subject matter experts


