Question: Kindly help with Question 1 and 2 1. (a). Define the following terms and state the expresions that relates them (i). Specific Conductance (ii). Equivalent
Kindly help with Question 1 and 2
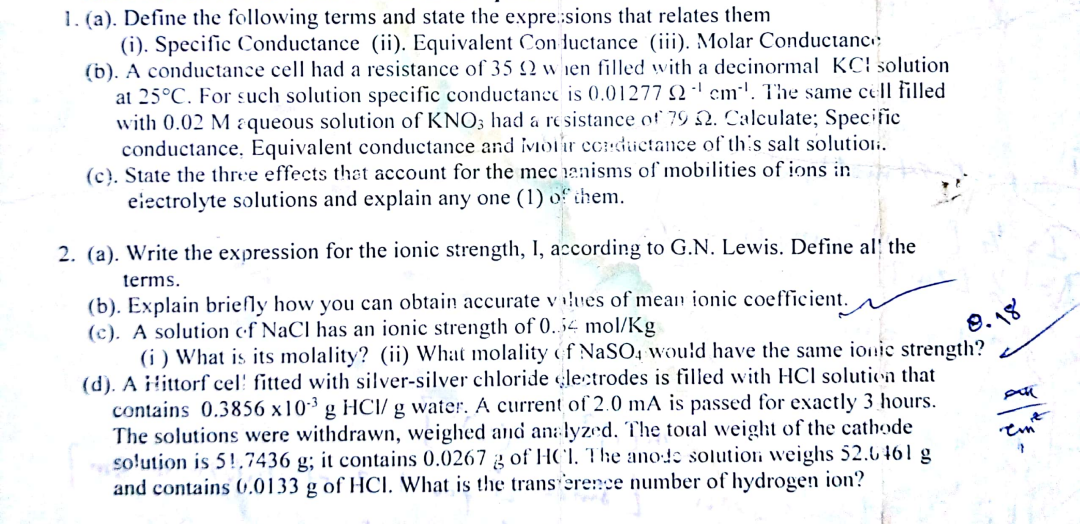
1. (a). Define the following terms and state the expresions that relates them (i). Specific Conductance (ii). Equivalent Conductance (iii). Molar Conductanc. (b). A conductance cell had a resistance of 352 when filled with a decinormal KCl solution at 25C. For such solution specific conductance is 0.01277 2 cm'. The same cell filled with 0.02 M aqueous solution of KNO3 had a resistance of 79 -2. Calculate; Specific conductance. Equivalent conductance and iviour ccrductance of this salt solutioli. (c). State the three effects that account for the mecanisms of mobilities of ions in electrolyte solutions and explain any one (1) of them. 9. 18 2. (a). Write the expression for the ionic strength, I, according to G.N. Lewis. Define all the terms. (b). Explain brielly how you can obtain accurate values of mean ionic coefficient. (c). A solution of NaCl has an ionic strength of 0.54 mol/Kg (i) What is its molality? (ii) What molality of NaSO, would have the same ionic strength? (d). A littorf cell fitted with silver-silver chloride electrodes is filled with HCl solution that contains 0.3856 x 10 g HCI/ g water. A current of 2.0 mA is passed for exactly 3 hours. 8 g The solutions were withdrawn, weighed and analyzed. The total weight of the cathode solution is 59.7436 g; it contains 0.0267 3 of HCl. The ano:!e solutiori weighs 52.6 461 g and contains 0,0133 g of HCl. What is the transference number of hydrogen ion? Pan tun 7
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


