Question: Lab 2 - Dry Lab - Determining the Molar Mass of the Unknown Acid - 5m Procedure: 1. A solution of 0.128mol/LNaOH was placed in
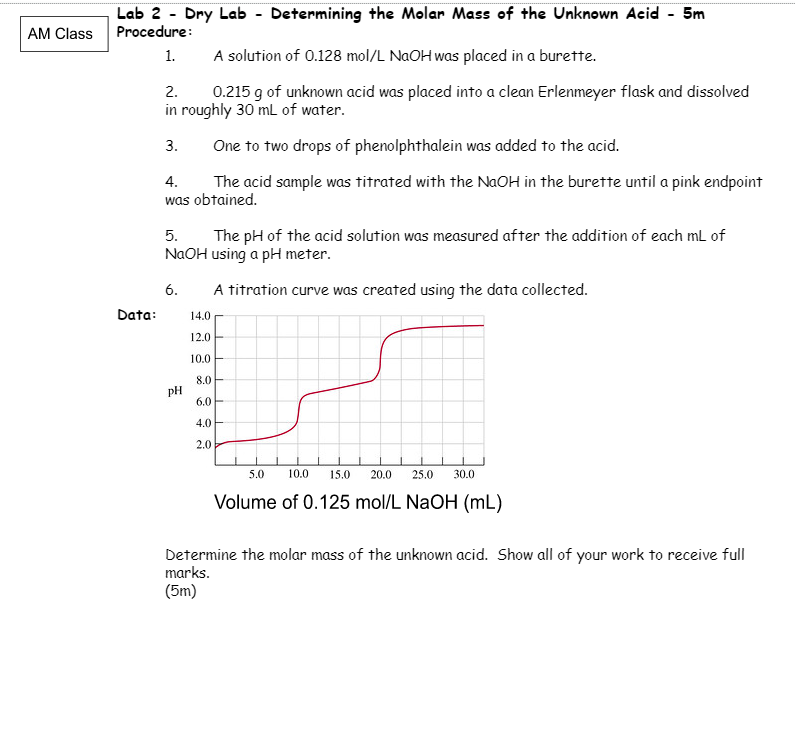
Lab 2 - Dry Lab - Determining the Molar Mass of the Unknown Acid - 5m Procedure: 1. A solution of 0.128mol/LNaOH was placed in a burette. 2. 0.215g of unknown acid was placed into a clean Erlenmeyer flask and dissolved in roughly 30mL of water. 3. One to two drops of phenolphthalein was added to the acid. 4. The acid sample was titrated with the NaOH in the burette until a pink endpoint was obtained. 5. The pH of the acid solution was measured after the addition of each mL of NaOH using a pH meter. 6. A titration curve was created using the data collected. Data: Determine the molar mass of the unknown acid. Show all of your work to receive full marks. (5m)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


