Question: PLEASE ANSWER ASAP: TWO PART QUESTION PART A: PART B: Complete the table below. Round each of your entries to 2 significant digits. You may
PLEASE ANSWER ASAP: TWO PART QUESTION
PART A:
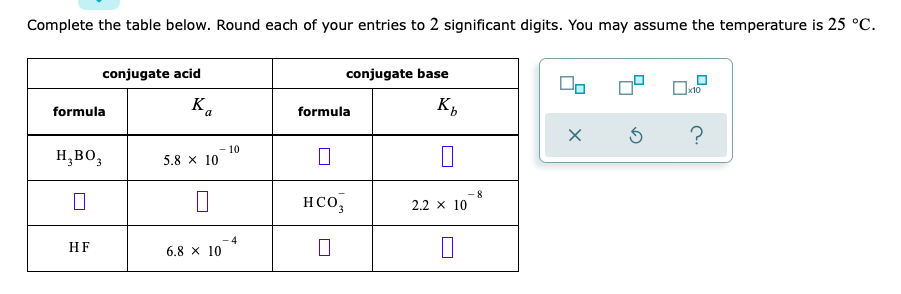
PART B:
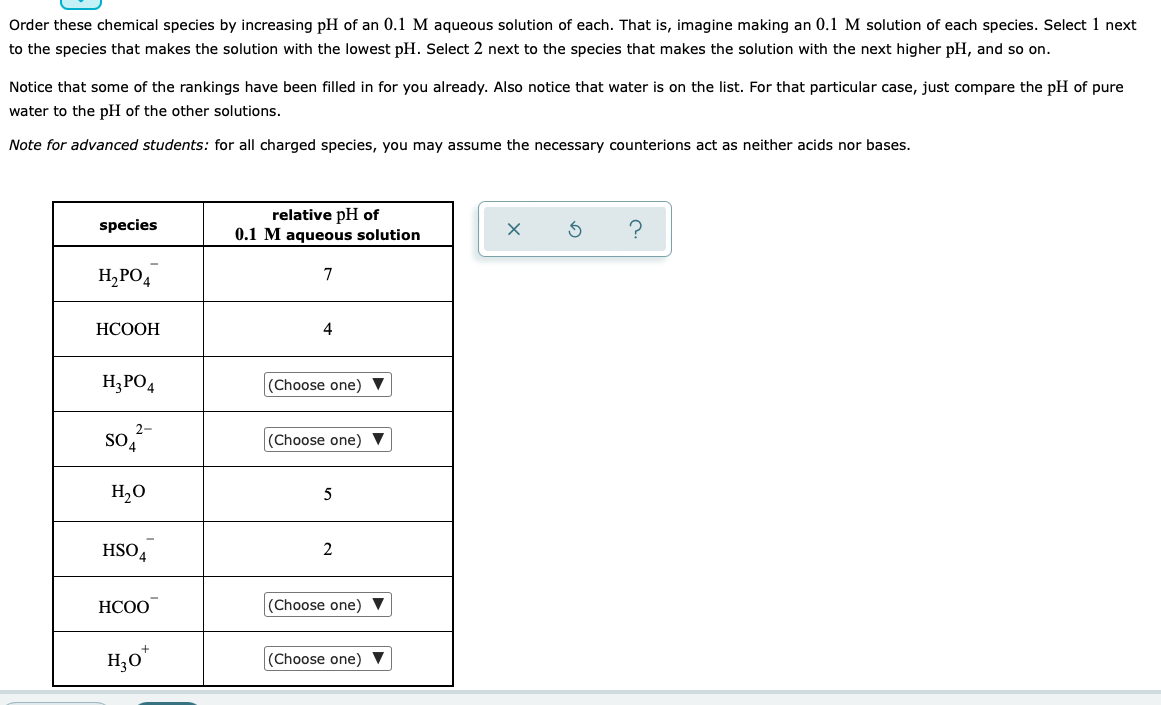
Complete the table below. Round each of your entries to 2 significant digits. You may assume the temperature is 25 C. conjugate acid conjugate base 0 formula Ka formula K 5 ? H,BOZ -10 5.8 x 10 8 HCO 2.2 x 10 HF 6.8 X 10 0 Order these chemical species by increasing pH of an 0.1 M aqueous solution of each. That is, imagine making an 0.1 M solution of each species. Select 1 next to the species that makes the solution with the lowest pH. Select 2 next to the species that makes the solution with the next higher pH, and so on. Notice that some of the rankings have been filled in for you already. Also notice that water is on the list. For that particular case, just compare the pH of pure water to the pH of the other solutions. Note for advanced students: for all charged species, you may assume the necessary counterions act as neither acids nor bases. species relative pH of 0.1 M aqueous solution $ ? H2PO4 7 HCOOH 4 H2PO4 (Choose one) 2- so,?- (Choose one) 7 HO 5 HSO4 2 HCOO (Choose one) H30* (Choose one)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


