Question: Lab Data Verify your volume measurement Did you report your data to the correct number of significant figures? Freezing-pointdepressionconstant(C/m)Freezing-pointofDIwater(C)1.860.30 Solution 1 Solution 2 Solution 3
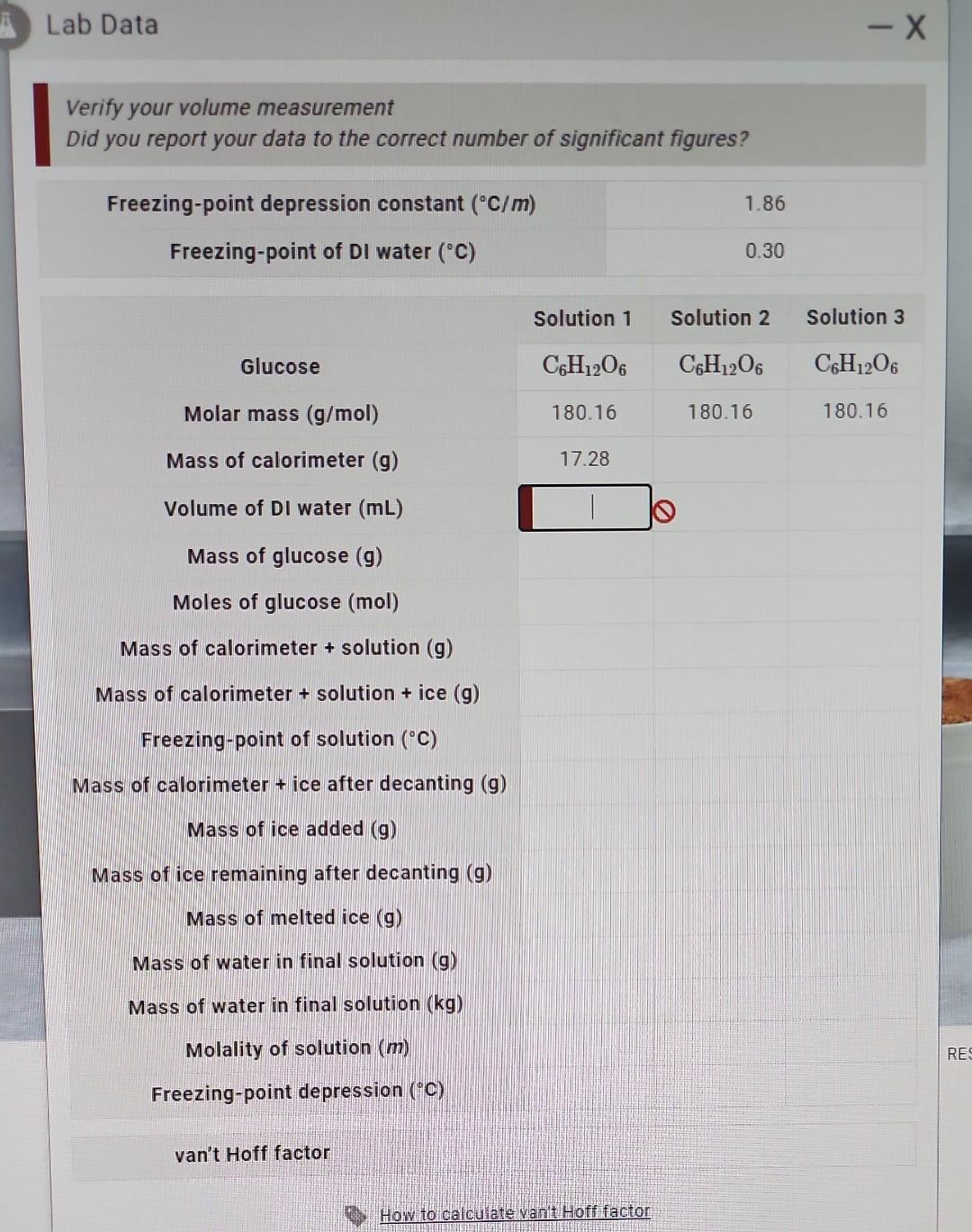
Lab Data Verify your volume measurement Did you report your data to the correct number of significant figures? Freezing-pointdepressionconstant(C/m)Freezing-pointofDIwater(C)1.860.30 Solution 1 Solution 2 Solution 3 \begin{tabular}{cccc} \multicolumn{1}{c}{ Glucose } & C6H12O6 & C6H12O6 & C6H12O6 \\ Molar mass (g/mol) & 180.16 & 180.16 & 180.16 \\ Mass of calorimeter (g) & 17.28 \\ Volume of DI water (mL) & & \\ \hline \end{tabular} Mass of glucose (g) Moles of glucose (mol) Mass of calorimeter + solution (g) Mass of calorimeter + solution + ice (g) Freezing-point of solution (C) Mass of calorimeter + ice after decanting (g) Mass of ice added (g) Mass of ice remaining after decanting (g) Mass of melted ice (g) Mass of water in final solution (g) Mass of water in final solution ( kg) Molality of solution (m) Freezing-point depression (C) van't Hoff factor How to calcuate vanit Hoff factor
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


