Question: Label the solid, liquid and gas in the phase diagram below: What is the boiling point of a 2.0mKCl(aq) solution? kb(H2O)=0.52C/m Enter your answer in
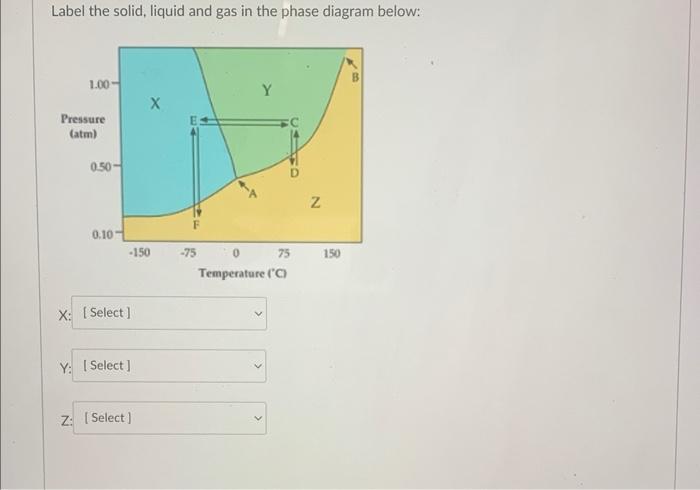
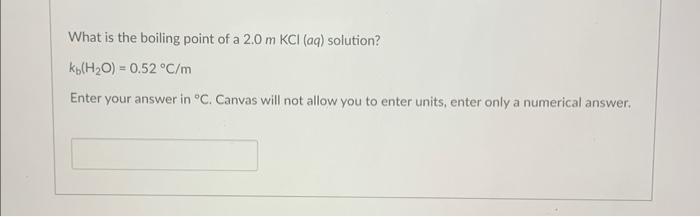
Label the solid, liquid and gas in the phase diagram below: What is the boiling point of a 2.0mKCl(aq) solution? kb(H2O)=0.52C/m Enter your answer in C. Canvas will not allow you to enter units, enter only a numerical
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


