Question: Last attempt and A and C are wrong. Pls help! = Consider the following set of quantum numbers: n = 2,1 = 1, m(t) =
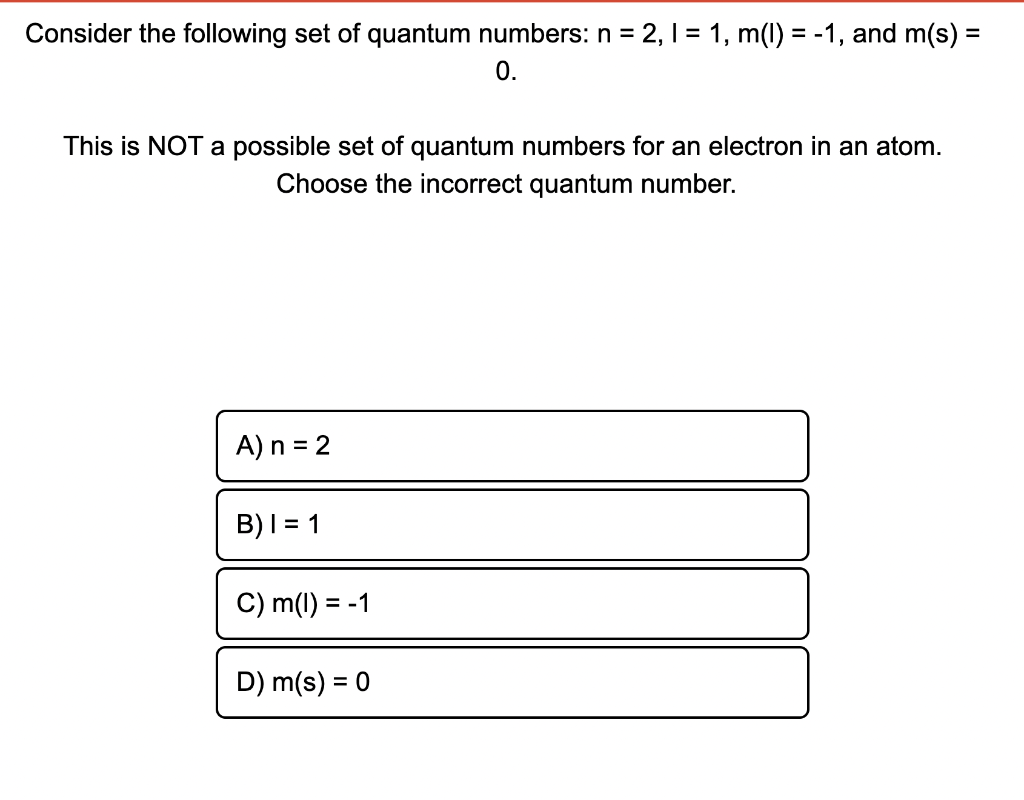
Last attempt and A and C are wrong. Pls help!
= Consider the following set of quantum numbers: n = 2,1 = 1, m(t) = -1, and m(s) = 0. This is NOT a possible set of quantum numbers for an electron in an atom. Choose the incorrect quantum number. A) n = 2 B) 1 = 1 C) m(t) = -1 D) m(s) = 0 =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


