Question: please help :) A researcher titrated a 100 ml solution of 0.1 m (fully protonated) glycine at pH 1.72 with 2 m NaOH solution. She
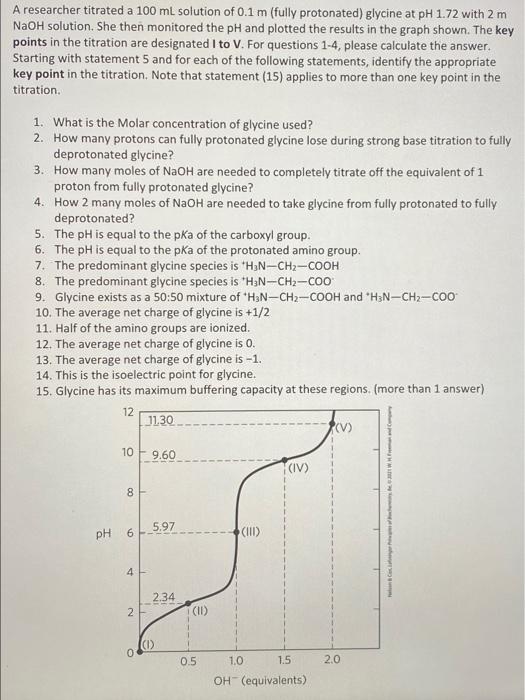
A researcher titrated a 100 ml solution of 0.1 m (fully protonated) glycine at pH 1.72 with 2 m NaOH solution. She then monitored the pH and plotted the results in the graph shown. The key points in the titration are designated I to V. For questions 1-4, please calculate the answer. Starting with statement 5 and for each of the following statements, identify the appropriate key point in the titration. Note that statement (15) applies to more than one key point in the titration. 1. What is the Molar concentration of glycine used? 2. How many protons can fully protonated glycine lose during strong base titration to fully deprotonated glycine? 3. How many moles of NaOH are needed to completely titrate off the equivalent of 1 proton from fully protonated glycine? 4. How 2 many moles of NaOH are needed to take glycine from fully protonated to fully deprotonated? 5. The pH is equal to the pka of the carboxyl group. 6. The pH is equal to the pka of the protonated amino group. 7. The predominant glycine species is H3N-CH2-COOH 8. The predominant glycine species is H3N-CH2-Coo 9. Glycine exists as a 50:50 mixture of HaN-CH2-COOH and H3N-CH2-COO 10. The average net charge of glycine is +1/2 11. Half of the amino groups are ionized. 12. The average net charge of glycine is 0. 13. The average net charge of glycine is -1. 14. This is the isoelectric point for glycine. 15. Glycine has its maximum buffering capacity at these regions. (more than 1 answer) 12 11.30 10 9.60 (IV) WH 00 8 PH 6 5.97 (III) 4 2.34 2 (II) (1) 0 0.5 1.0 1.5 2.0 OH-(equivalents) A researcher titrated a 100 ml solution of 0.1 m (fully protonated) glycine at pH 1.72 with 2 m NaOH solution. She then monitored the pH and plotted the results in the graph shown. The key points in the titration are designated I to V. For questions 1-4, please calculate the answer. Starting with statement 5 and for each of the following statements, identify the appropriate key point in the titration. Note that statement (15) applies to more than one key point in the titration. 1. What is the Molar concentration of glycine used? 2. How many protons can fully protonated glycine lose during strong base titration to fully deprotonated glycine? 3. How many moles of NaOH are needed to completely titrate off the equivalent of 1 proton from fully protonated glycine? 4. How 2 many moles of NaOH are needed to take glycine from fully protonated to fully deprotonated? 5. The pH is equal to the pka of the carboxyl group. 6. The pH is equal to the pka of the protonated amino group. 7. The predominant glycine species is H3N-CH2-COOH 8. The predominant glycine species is H3N-CH2-Coo 9. Glycine exists as a 50:50 mixture of HaN-CH2-COOH and H3N-CH2-COO 10. The average net charge of glycine is +1/2 11. Half of the amino groups are ionized. 12. The average net charge of glycine is 0. 13. The average net charge of glycine is -1. 14. This is the isoelectric point for glycine. 15. Glycine has its maximum buffering capacity at these regions. (more than 1 answer) 12 11.30 10 9.60 (IV) WH 00 8 PH 6 5.97 (III) 4 2.34 2 (II) (1) 0 0.5 1.0 1.5 2.0 OH-(equivalents)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


