Question: Lets consider a container with a liquid A at room temperature and atmospheric pressure. We are interested in calculating the flux O2 from air into
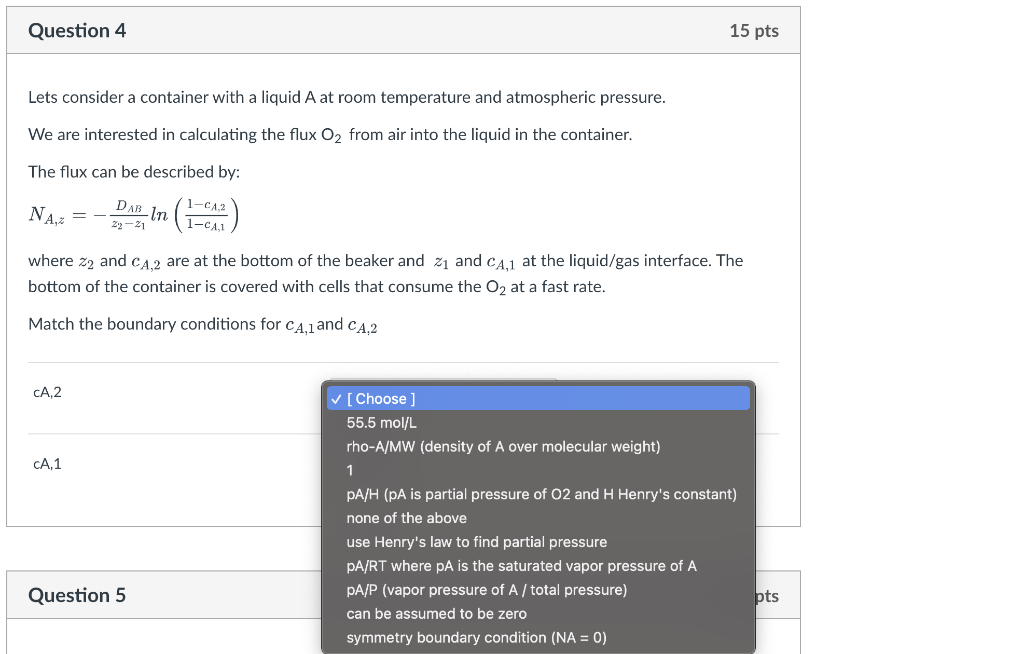
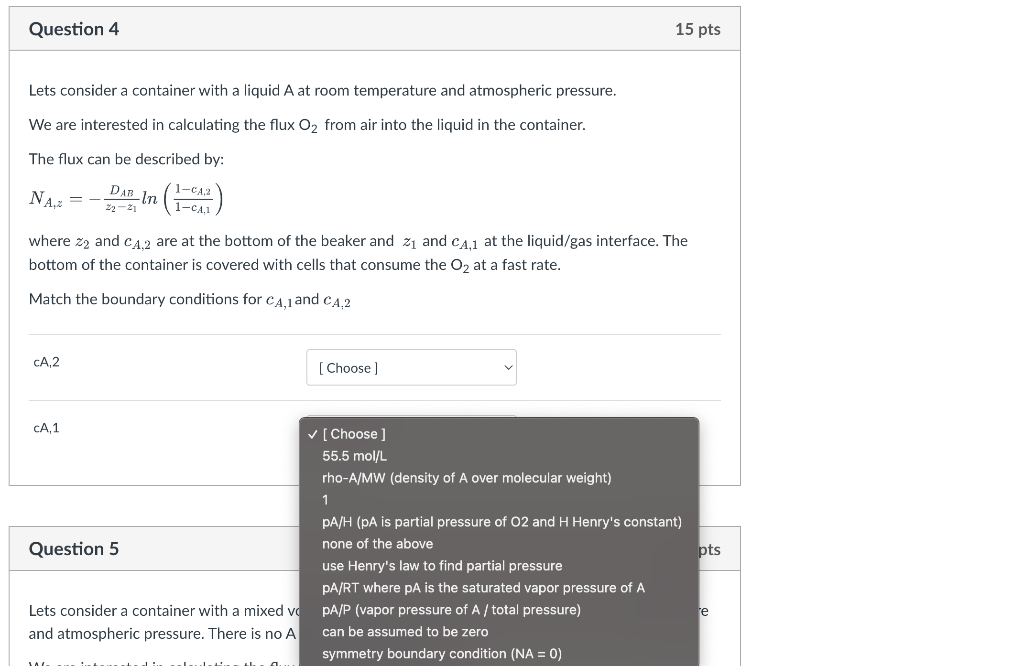
Lets consider a container with a liquid A at room temperature and atmospheric pressure. We are interested in calculating the flux O2 from air into the liquid in the container. The flux can be described by: NA,z=z2z1DABln(1cA,11cA,2) where z2 and cA,2 are at the bottom of the beaker and z1 and cA,1 at the liquid/gas interface. The bottom of the container is covered with cells that consume the O2 at a fast rate. Match the boundary conditions for cA,1 and cA,2 Lets consider a container with a liquid A at room temperature and atmospheric pressure. We are interested in calculating the flux O2 from air into the liquid in the container. The flux can be described by: NA,z=z2z1DABln(1cA,11cA,2) where z2 and cA,2 are at the bottom of the beaker and z1 and cA,1 at the liquid/gas interface. The bottom of the container is covered with cells that consume the O2 at a fast rate. Match the boundary conditions for cA,1 and cA,2 cA,2 cA,1 [ Choose ] 55.5mol/L rho-A/MW (density of A over molecular weight) 1 pA/H (pA is partial pressure of O2 and H Henry's constant) Question 5 none of the above use Henry's law to find partial pressure PA/RT where PA is the saturated vapor pressure of A Lets consider a container with a mixed VopA/P (vapor pressure of A/ total pressure) and atmospheric pressure. There is no A can be assumed to be zero symmetry boundary condition (NA=0)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


