Question: Lewis dot structures have three general exceptions to the octet rule, one of whicl Molecules in which one or more atoms possess more than eight
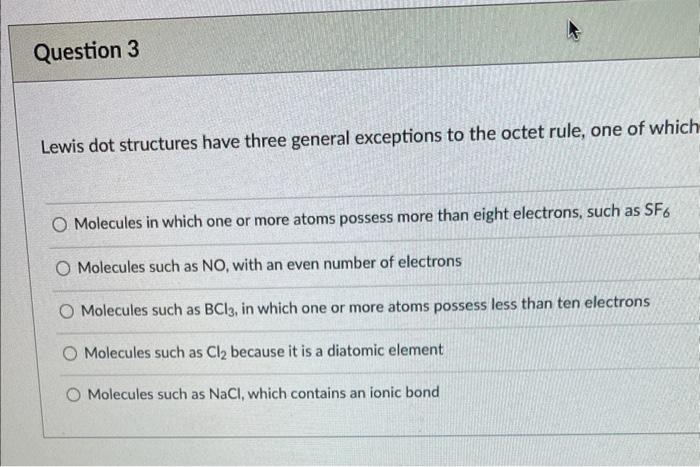
Lewis dot structures have three general exceptions to the octet rule, one of whicl Molecules in which one or more atoms possess more than eight electrons, such as SF6 Molecules such as NO, with an even number of electrons Molecules such as BCl3, in which one or more atoms possess less than ten electrons Molecules such as Cl2 because it is a diatomic element Molecules such as NaCl, which contains an ionic bond
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


