Question: Light emitted from a source passes through a sample. If the sample is capable of absorbir that light, it will not reach the detector. The
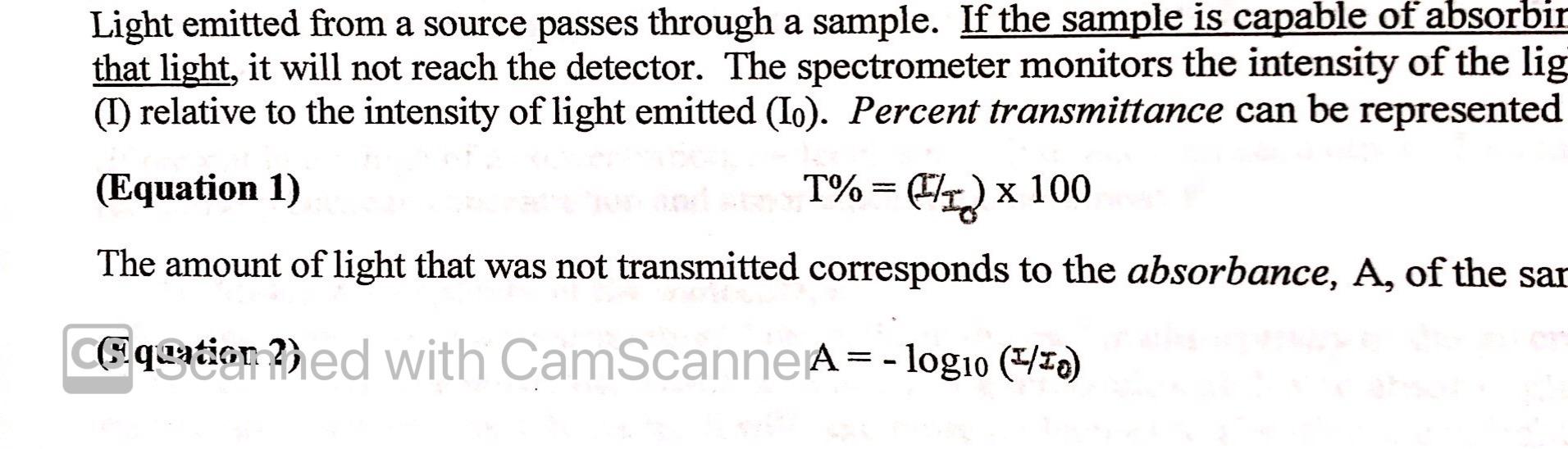

Light emitted from a source passes through a sample. If the sample is capable of absorbir that light, it will not reach the detector. The spectrometer monitors the intensity of the lig (1) relative to the intensity of light emitted (lo). Percent transmittance can be represented (Equation 1) T%= q) x 100 1/) The amount of light that was not transmitted corresponds to the absorbance, A, of the sar Equation 2) ed with CamScanne A = - log10 (4/10) 4. For a particular molecule, a Beer's Law plot generated from absorption spectroscopy has a best fit line with equation y= 31008 x. What would be the concentration (Units would be M or mol/L) of a solution of this molecule that registers absorbance of 0.44? Show your work. 5. Compare Equation 1 and 2 in the Introduction. Calculate the % transmittance for a solution that has absorbance of 0.44? Show your work
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


