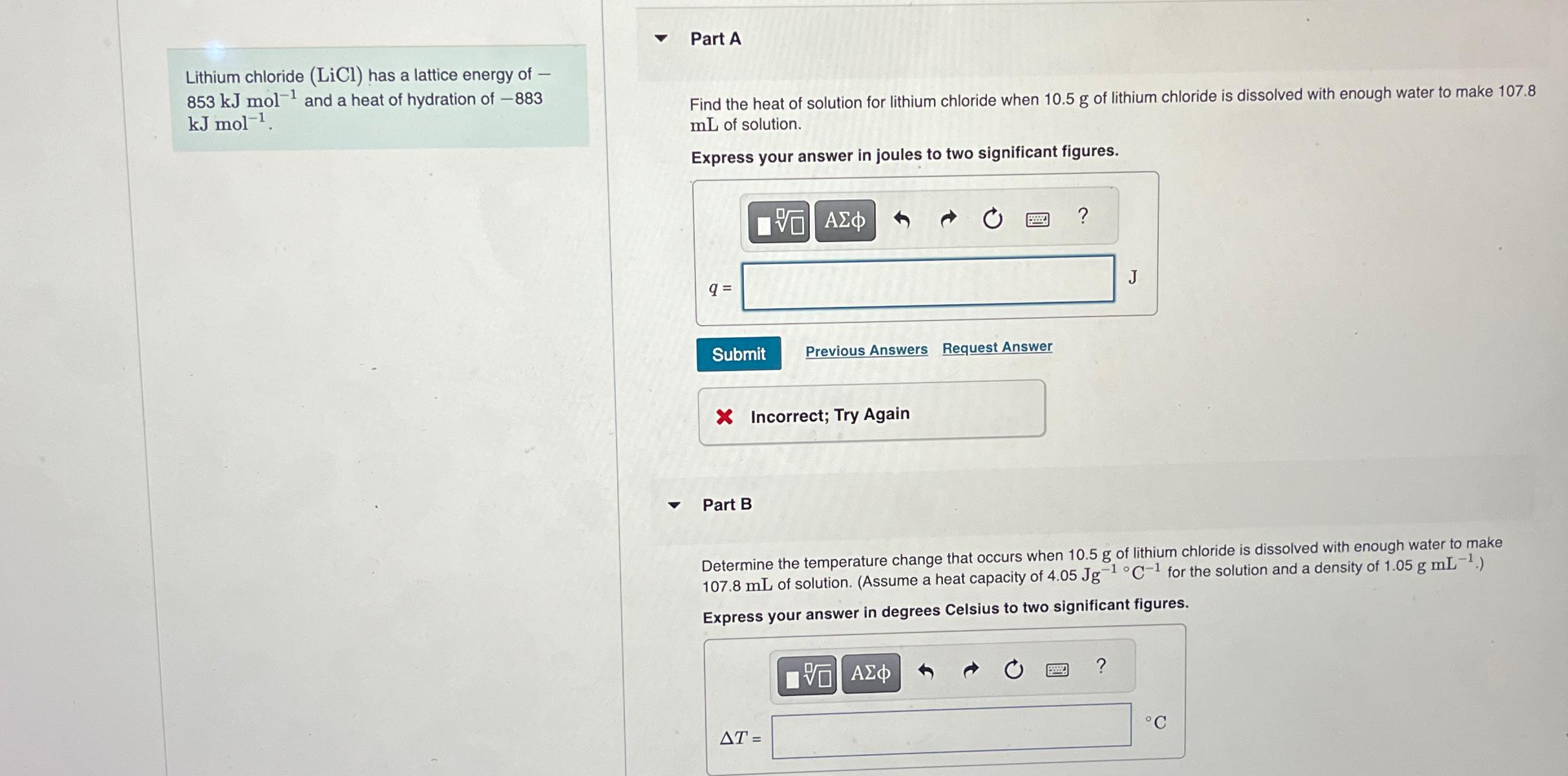
Question: Lithium chloride ( L i C l ) has a lattice energy of 8 5 3 k J m o l - 1 and a
Lithium chloride has a lattice energy of and a heat of hydration of
Part A
Find the heat of solution for lithium chloride when of lithium chloride is dissolved with enough water to make of solution.
Express your answer in joules to two significant figures.
J
Previous Answers
Request Answer
Incorrect; Try Again
Part B
Determine the temperature change that occurs when of lithium chloride is dissolved with enough water to make of solution. Assume a heat capacity of for the solution and a density of
Express your answer in degrees Celsius to two significant figures.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


