Question: LO 4 . 2 + 4 . 4 - Q 1 Instead of looking at the kinetics of a drug interacting with an enzyme, you
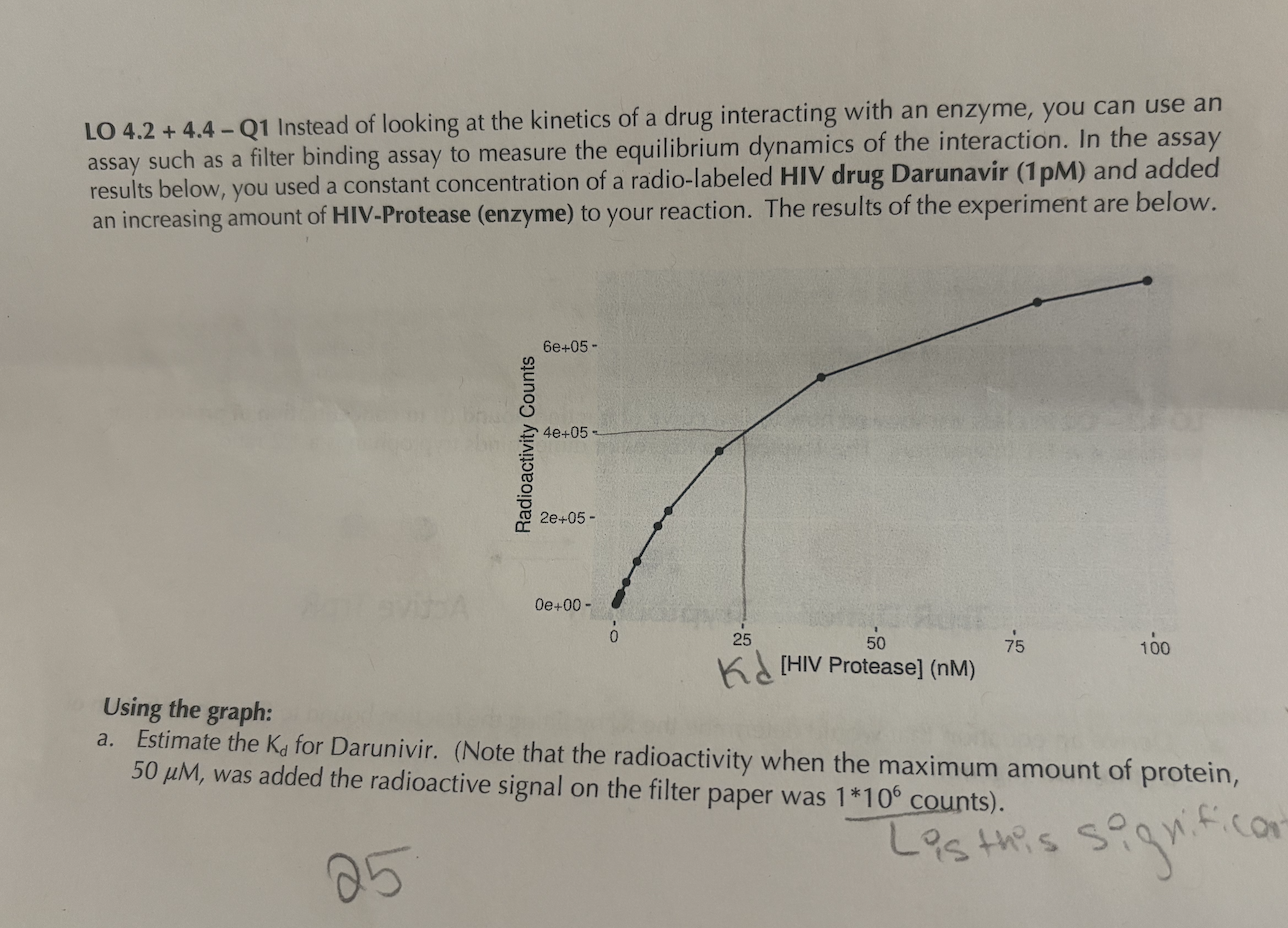
LO Q Instead of looking at the kinetics of a drug interacting with an enzyme, you can use an assay such as a filter binding assay to measure the equilibrium dynamics of the interaction. In the assay results below, you used a constant concentration of a radiolabeled HIV drug Darunavir pM and added an increasing amount of HIVProtease enzyme to your reaction. The results of the experiment are below.
Using the graph:
a Estimate the Kd for Darunivir. Note that the radioactivity when the maximum amount of protein, mu mathrmM was added the radioactive signal on the filter paper was counts b Calculate the Delta Gtext bind o for the interaction between HIV protease and Darunivir at K K
y
beginarrayl
GR T ln K R
endarray
Binding of a drug to a protein is entropically unfavorable molecules bind to form molecule
Yestertion is VERY favorable. What are two ways that Darunivirbinding could overcome the large entropic unfavourability of binding for a net favorable reaction. Be specific.
Entropic Increase in Binding of the solvent that carraes it
Enthalpic increase in Binding
The enthalpic encmease cap cone due to string Hbonds cleate cavoialde enthal pac rracractions
d Sketch a Hill Plot of the Darunivir binding to HIV Protease Data. Indicate what the slope, Xintercept, and Yintercept would be
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


