Question: Logarithms and Logarithmic Function Practice Problem Chapter 7 - Logarithms and Logarithmic Functions 20. In Chemistry, the PH of a substance can be determined using
Logarithms and Logarithmic Function
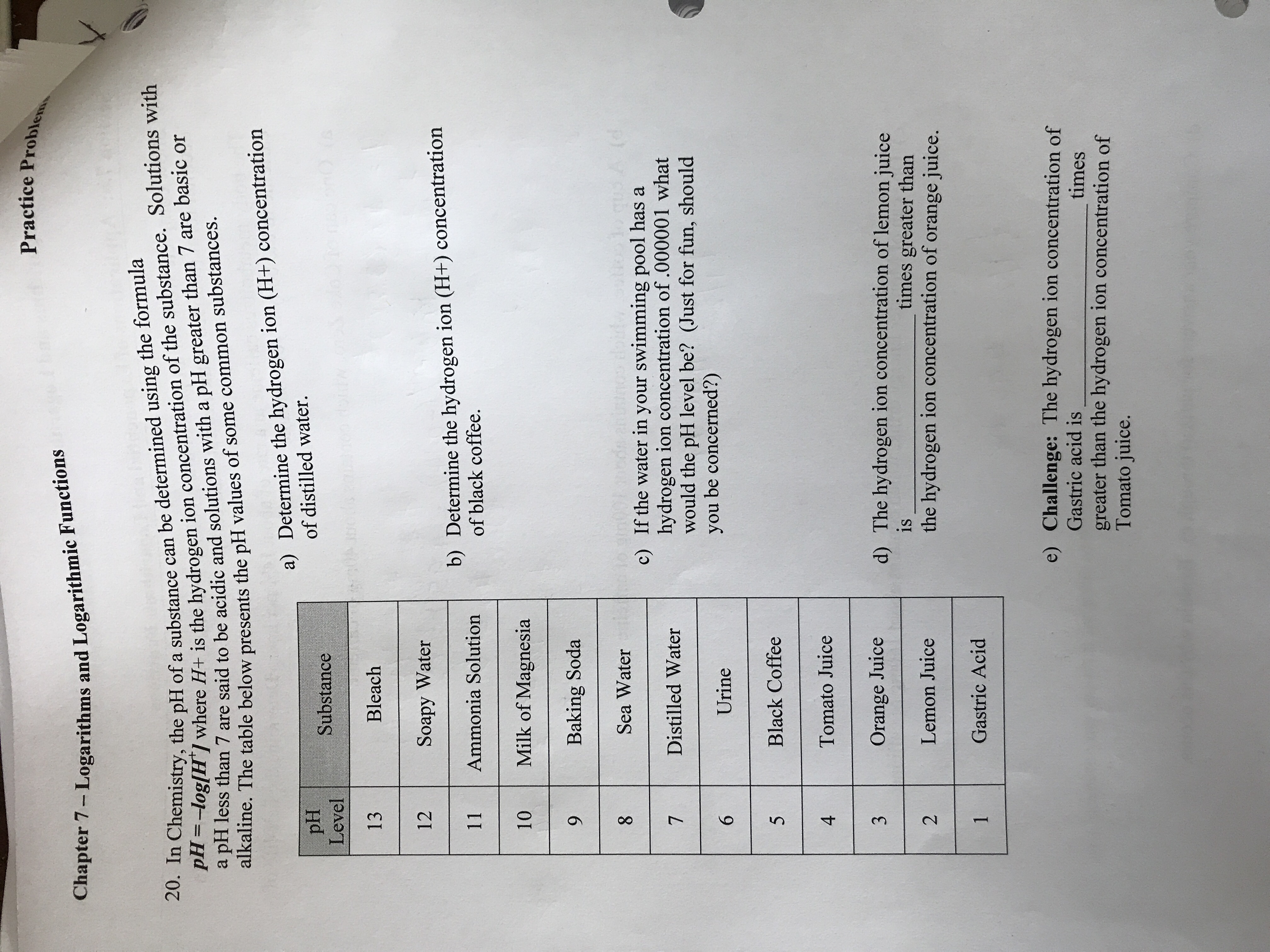
Practice Problem Chapter 7 - Logarithms and Logarithmic Functions 20. In Chemistry, the PH of a substance can be determined using the formula PH =-log[H / where H+ is the hydrogen ion concentration of the substance. Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline. The table below presents the pH values of some common substances. a) Determine the hydrogen ion (H+) concentration PH Substance of distilled water. Level 13 Bleach 12 Soapy Water 11 b) Determine the hydrogen ion (H+) concentration Ammonia Solution of black coffee. 10 Milk of Magnesia 9 Baking Soda Sea Water c) If the water in your swimming pool has a Distilled Water hydrogen ion concentration of .000001 what would the pH level be? (Just for fun, should 6 Urine you be concerned?) 5 Black Coffee 4 Tomato Juice 3 Orange Juice d) The hydrogen ion concentration of lemon juice is 2 Lemon Juice times greater than the hydrogen ion concentration of orange juice. Gastric Acid e) Challenge: The hydrogen ion concentration of Gastric acid is greater than the hydrogen ion concentration of times Tomato juice
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


