Question: Look at the figure in below and answer these question according to it. a) How many atoms does this cubic structure have? (5 points) b)
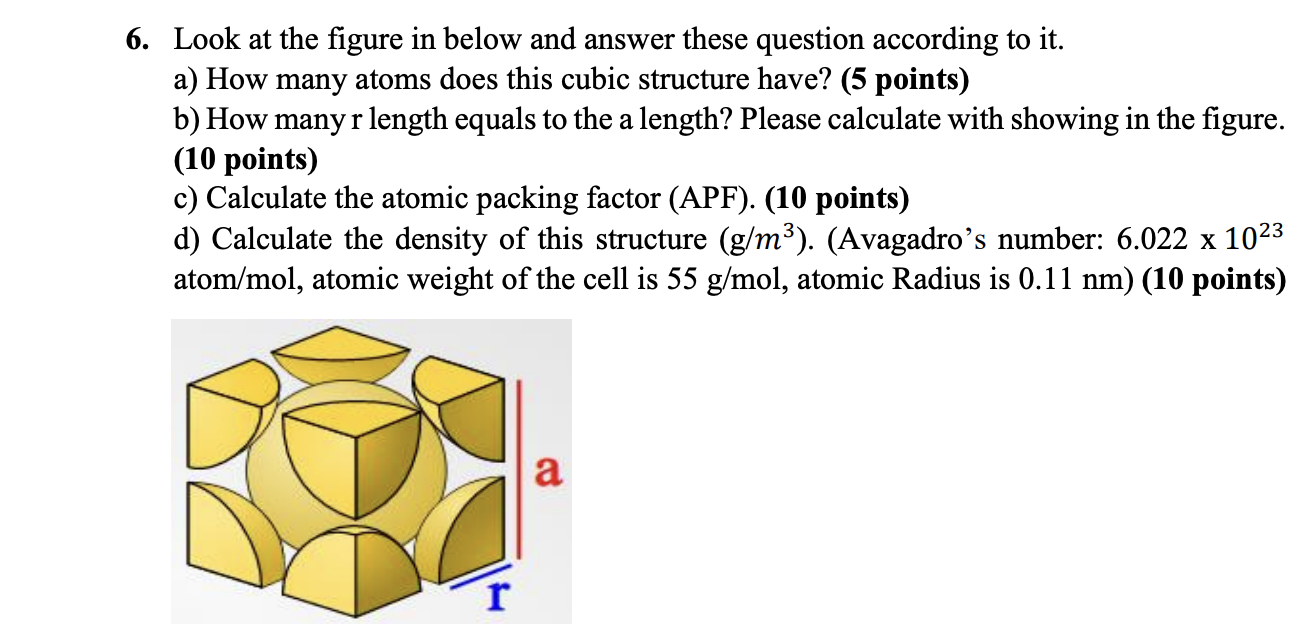
Look at the figure in below and answer these question according to it. a) How many atoms does this cubic structure have? (5 points) b) How many r length equals to the a length? Please calculate with showing in the figure. (10 points) c) Calculate the atomic packing factor (APF). (10 points) d) Calculate the density of this structure (g/m3). (Avagadro's number: 6.0221023 atom /mol, atomic weight of the cell is 55g/mol, atomic Radius is 0.11nm) (10 points)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


