Question: Make a step by step process in this problem in words. Q.6 Gas is expanding P=qv2 Vi Here , V- = $ 1 m3 -
Make a step by step process in this problem in words.
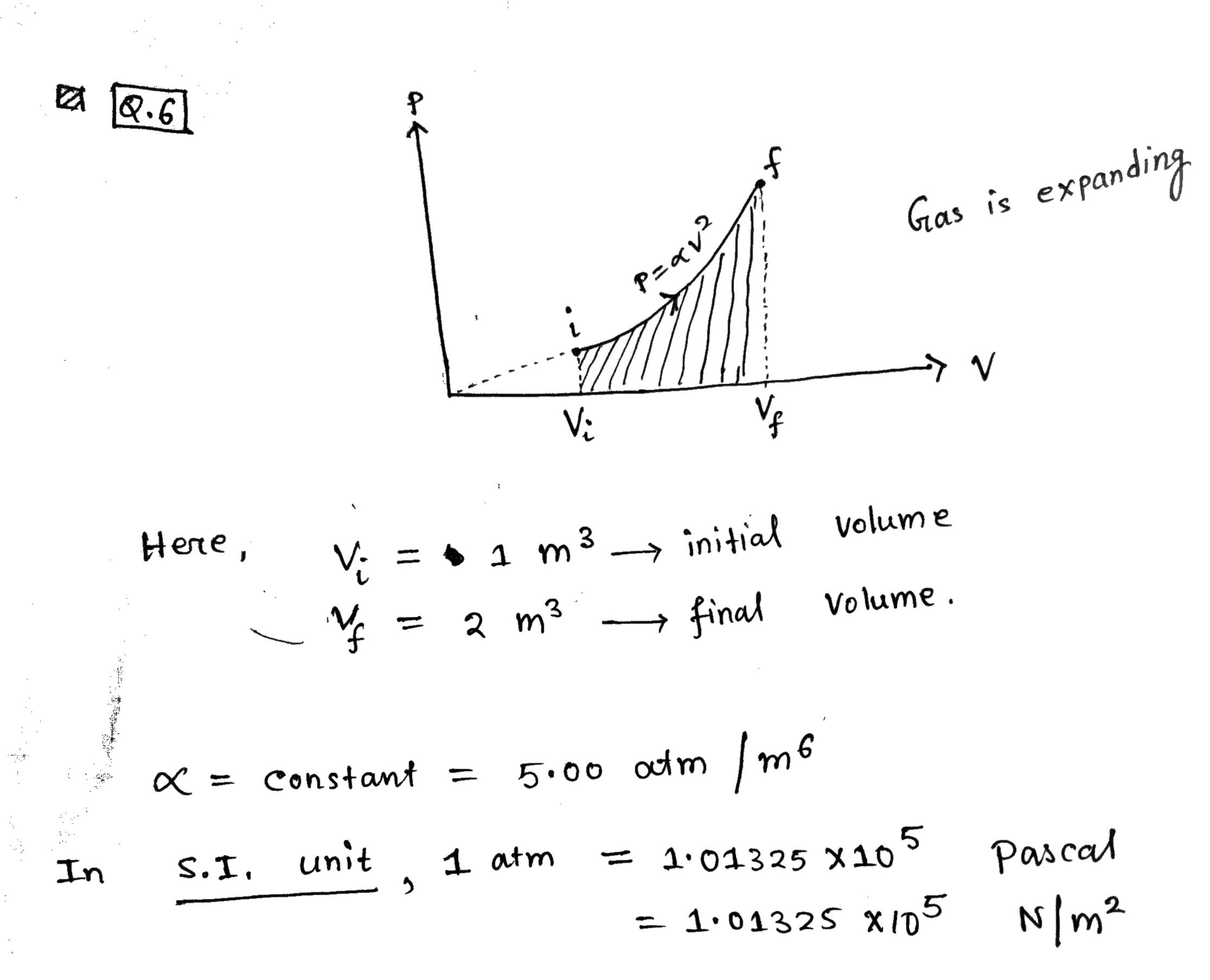

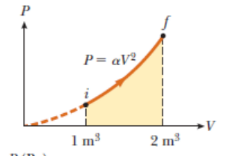
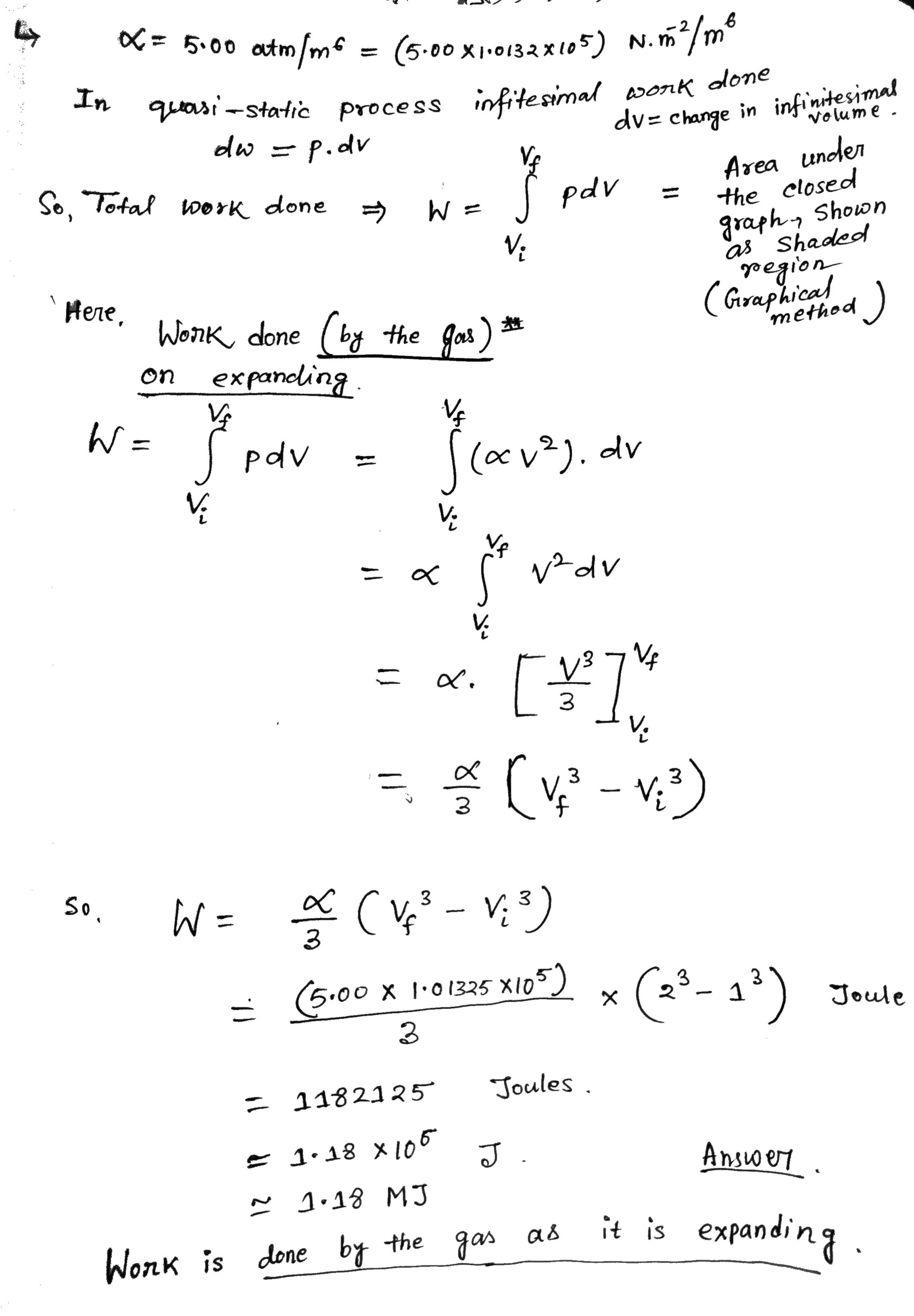
Q.6 Gas is expanding P=qv2 Vi Here , V- = $ 1 m3 - initial volume = 2 m3 -final Volume . X = constant = 5:00 atm / m6 In S. I, unit 1 atm = 101325 x10 Pascal = 101325 x105 N/ m 26. A sample of ideal gas is expanded to twice its original volume of 1.00 m3 in a quasi-static process for which P=aV2, with a: 5.00 arm/m5 as shown in the gure. How much work is clone on the expanding gas? P = QV2 1 m m* = 5.00 atm /me = ( 5. 00 x1 013 2 x105 ) N. m2/ me In quasi-static process infitesimal work done dv = change in infinitesimal volume . odw = p. dv Vf Area under So , Total work done =) pdv W = = the closed graphy Shown V: as Shaded region Here, ( Graphical Work done ( by the Jous ) * method on expanding Vf W = pov = ( ov 2). dv Ve V 2 d v V- w
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


