Question: Make sure answer for part d is both A and B seperated with a coma Write the rate law for the reaction. begin{tabular}{l|l} MISSED THIS?
Make sure answer for part d is both A and B seperated with a coma
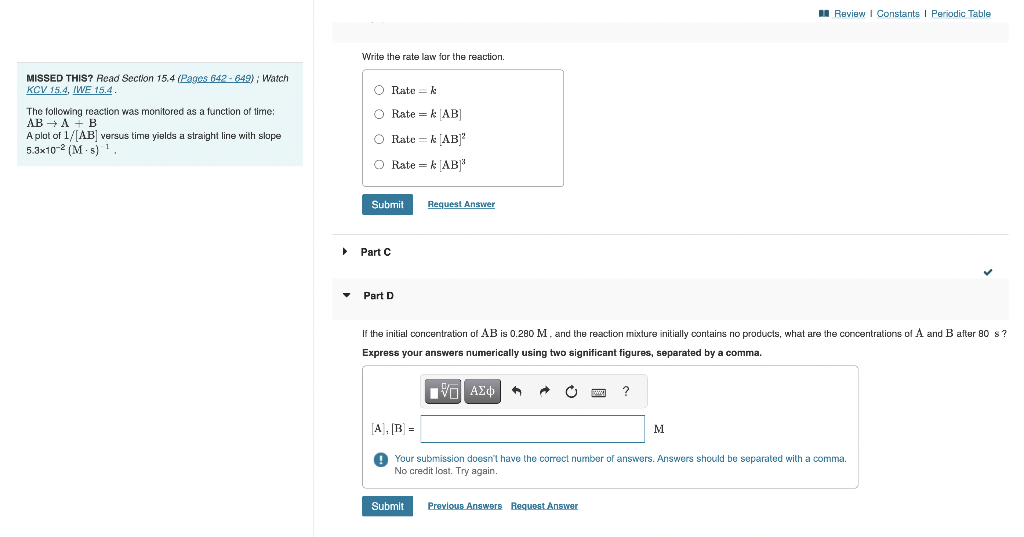
Write the rate law for the reaction. \begin{tabular}{l|l} MISSED THIS? Read Section 15.4, & iWatch \\ KCV 15.4, & Rate =k \\ The following reaction was monitored as a function of time: & Rate =k[AB] \\ ABA+B & \\ A plot of 1/[AB] versus time yields a straight line with slope \\ 5.3102(M5)1. & Rate =k[AB]2 \\ & Rate =k[AB]3 \end{tabular} - Part C - Part D If the initial concentration of AB is 0.280M, and the reaction mixture initially contains no products, what are the concentrations of A and B after 80s ? Express your answers numerically using two significant figures, separated by a comma
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


