Question: What is the half-life when the initial concentration is 0.50M ? MISSED THIS? Watch KCV : The Integrated Rate Law, Express your answer in seconds
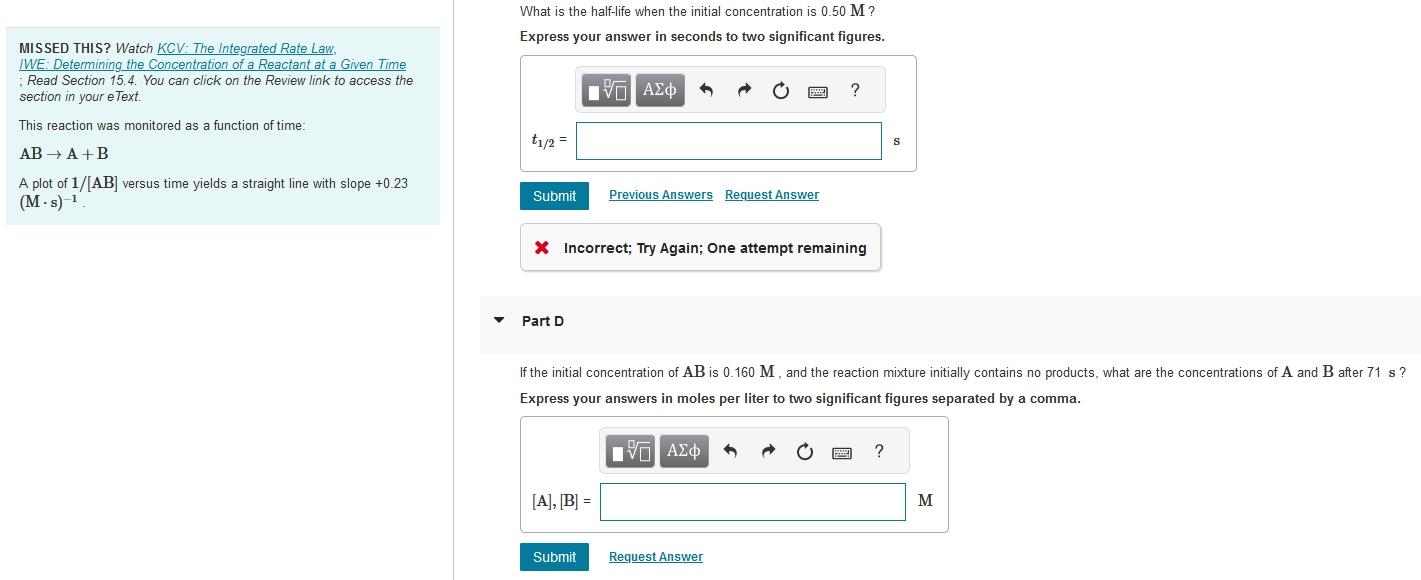
What is the half-life when the initial concentration is 0.50M ? MISSED THIS? Watch KCV : The Integrated Rate Law, Express your answer in seconds to two significant figures. IWE: Determining the Concentration of a Reactant at a Given Time ; Read Section 15.4. You can click on the Review link to access the section in your eText. This reaction was monitored as a function of time: ABA+B A plot of 1/[AB] versus time yields a straight line with slope +0.23 (Ms)1 Incorrect; Try Again; One attempt remaining Part D If the initial concentration of AB is 0.160M, and the reaction mixture initially contains no products, what are the concentrations of A and B after 71 s ? Express your answers in moles per liter to two significant figures separated by a comma
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


