Question: MASS SPECTRUM Consider the above spectrum. How do you interpret the size of the peaks provided by the y axis of an MS spectrum? The
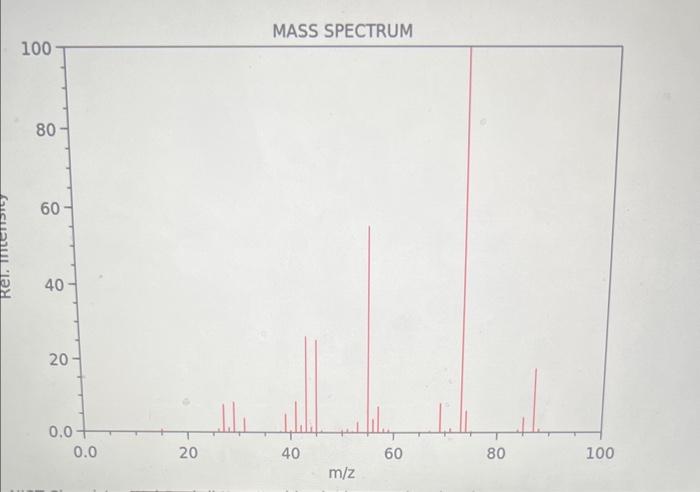

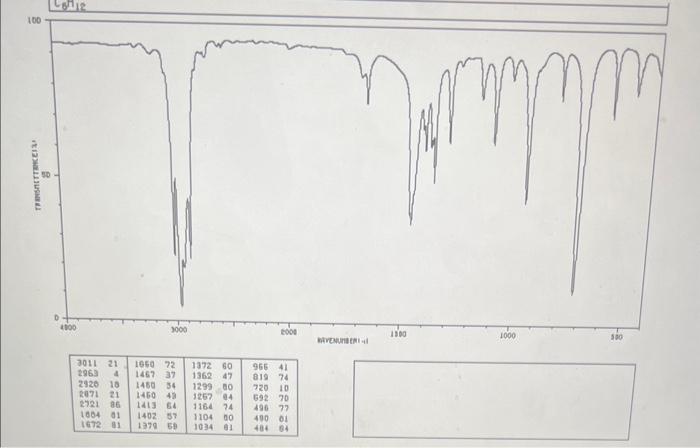
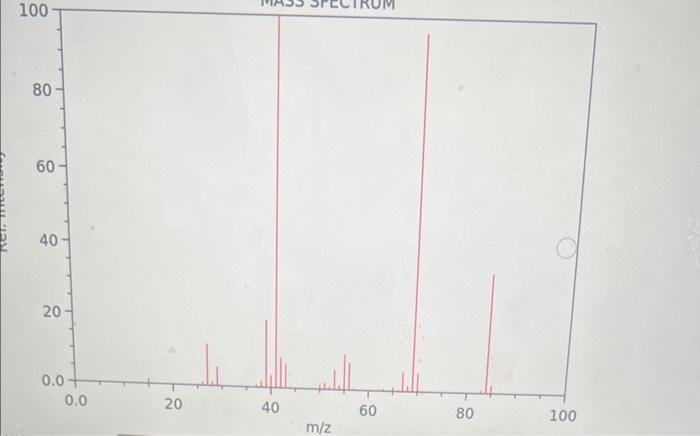

MASS SPECTRUM Consider the above spectrum. How do you interpret the size of the peaks provided by the y axis of an MS spectrum? The tallest the peak, the less stable the ion that causes it The shortest the peak, the more stable the ion that causes it The tallest the peak, the more stable the ion that causes it What type of compound do the above IR and MS spectra represent? Justify your answer. Comparison with the saturated CnH(2n+2) reveals that C6H12 is missing 2H 's, while the sharp and medium size peak at 3011rcm on the IR spectrum indicative of the vibration of a HC that is part of C=C bond vibration. Therefore the molecule is a cyclic alkene. Comparison with the saturated CnH(2n) reveals that C6H12 is missing 0 H's, while the sharp and medium size peak at 2963rcm on the IR spectrum indicative of a C=C bond vibration. Therefore the molecule is an open chain alkane. Comparison with the saturated CnH(2n+2) reveals that C6H12 is missing 2H 's, while the sharp and medium size peak at 1660rcm on the IR spectrum indicative of a C=C bond vibration. Therefore the molecule is an open chain alkene. MASS SPECTRUM Consider the above spectrum. How do you interpret the size of the peaks provided by the y axis of an MS spectrum? The tallest the peak, the less stable the ion that causes it The shortest the peak, the more stable the ion that causes it The tallest the peak, the more stable the ion that causes it What type of compound do the above IR and MS spectra represent? Justify your answer. Comparison with the saturated CnH(2n+2) reveals that C6H12 is missing 2H 's, while the sharp and medium size peak at 3011rcm on the IR spectrum indicative of the vibration of a HC that is part of C=C bond vibration. Therefore the molecule is a cyclic alkene. Comparison with the saturated CnH(2n) reveals that C6H12 is missing 0 H's, while the sharp and medium size peak at 2963rcm on the IR spectrum indicative of a C=C bond vibration. Therefore the molecule is an open chain alkane. Comparison with the saturated CnH(2n+2) reveals that C6H12 is missing 2H 's, while the sharp and medium size peak at 1660rcm on the IR spectrum indicative of a C=C bond vibration. Therefore the molecule is an open chain alkene
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


