Question: Mass Transport Operations Question 1 : A tray tower is to be used to remove 9 4 % of the ammonia from an entering air
Mass Transport Operations
Question :
A tray tower is to be used to remove of the ammonia from an entering air stream containing mol ammonia at and The inert air flow rate is inert air
Determine the minimum solvent flow ie pure water rate for the required separation,
Using determine the required solvent flowrate
Determine the compositions of the solvent and the gas phase leaving the absorber
Calculate the number of theoretical trays needed using the graphical method
Compare your results using the analytical Kremer method
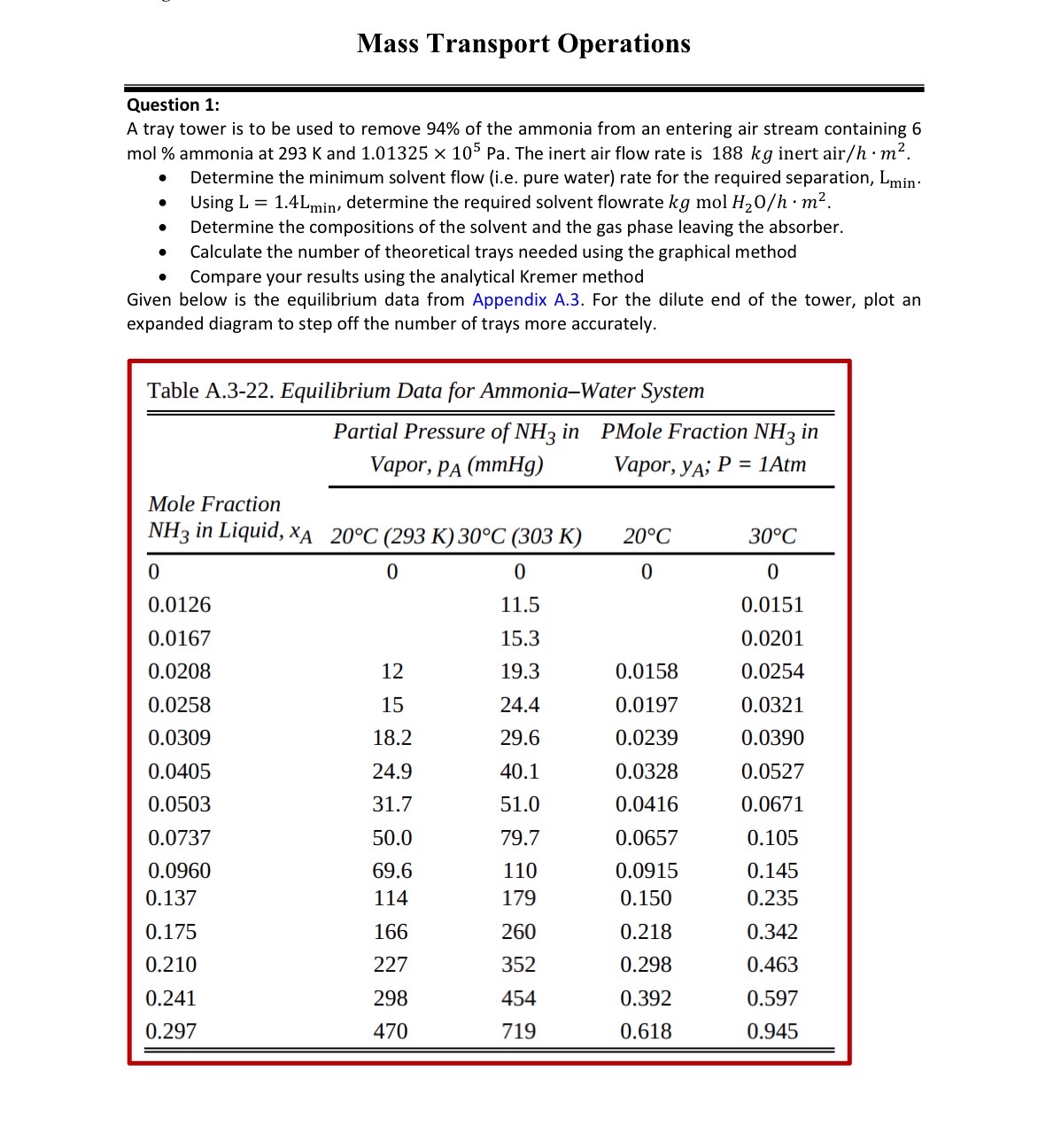
Given below is the equilibrium data from Appendix A For the dilute end of the tower, plot an expanded diagram to step off the number of trays more accurately.
tableTable A Equilibrium Data for AmmoniaWater SystemtableMole Fraction in Liquid,
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


