Question: 32 grams of (C,H) is placed in a container with 39 grams of oxygen gas. 28 grams of CO, are collected at the end

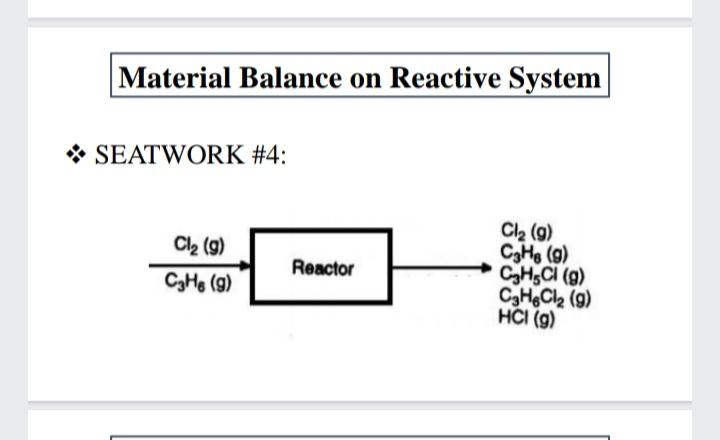

32 grams of (C,H) is placed in a container with 39 grams of oxygen gas. 28 grams of CO, are collected at the end of the reaction. Calculate the percent yield. Material Balance on Reactive System * SEATWORK # 4: We have two reactions: Cl2 (g) + CsH6 (g) CHSCI (g) +HCI Cl: (g) + CsH, (g) C,H,Cl; (g) () (b) The species recovered after the reaction takes place for some time are listed in table below: Species Cl, CH6. propylene CH,CI, ally chloride C,H,Cl, propylene chloride MW gmol 141.0 651.0 42.08 76.53 112.99 4.6 24.5 HCI 4.6 Material Balance on Reactive System * SEATWORK # 4: Based on the product distribution assuming that no ally chlorides were present in the feed, calculate the following: a. How much Cl2 and C3H6 were fed to the reactor in gmol? b. What was the limiting reactant? c. What was the excess reactant? d. What was the fraction conversion of C,H6 to C3H;C1? e. What was the selectivity of C3H3C1 relative to C3H,C12? f. What was the yield of C3H;CI expressed in g of C;H;CI to the g of C;H, fed to the reactor? g. What was the extent of reaction of the first and second reactions? Material Balance on Reactive System * SEATWORK #4: Cl2 (g) CHe (g) CH,CI (g) CaHeCl2 (9) HCI (g) Cl2 (g) Reactor CHe (g) Estimation of Vapor Pressures SEATWORK #1: Calculate the grams of glucose that must be added to 2.85 kg of H2O at 65 degrees Celsius to lower the vapor pressure by 10.0 torr if the vapor pressure of pure water at this temperature is 188 torr.
Step by Step Solution
3.51 Rating (151 Votes )
There are 3 Steps involved in it
Q1 Solu... View full answer

Get step-by-step solutions from verified subject matter experts


