Question: MATLAB Coding Problem You are to develop an interactive computer program (Matlab or any computer programming code) on fuel combustion. Your program should balance the
MATLAB Coding Problem
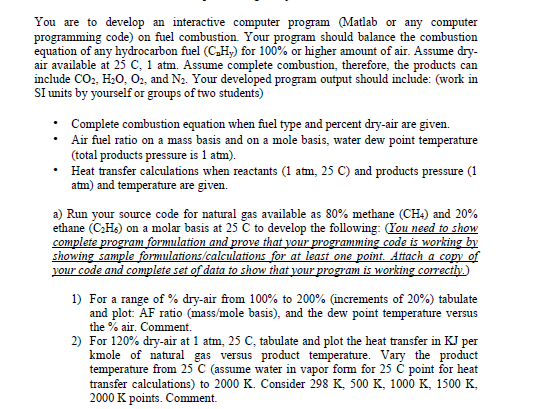
You are to develop an interactive computer program (Matlab or any computer programming code) on fuel combustion. Your program should balance the combustioin equation of any hydrocarbon fuel (CDH,) for 100% or higher amount of air. Assume dry air available at 25 C, 1 atm. Assume complete combustion, therefore, the products carn include CO2, H20, O2, and N2. Your developed program output should include: (work in SI units by yourself or groups of two students) Complete combustion equation when fuel type and percent dry-air are given (total products pressure is 1 atm). atm) and temperature are given. *Air fuel ratio on a mass basis and on a mole basis, water dew point temperature Heat transfer calculations when reactants (1 atm, 25 C) and products pressure (1 a) Run your source code for natural gas available as 80% methane (CH4) and 20% ethane (C2Hs) on a molar basis at 25 C to develop the following: You need to show complete showing sample formulations/calculations for at your ammi ar your piogram 1) For a range of % dry-air from 100% to 200% (increments of 20%) tabulate and plot: AF ratio (mass/mole basis), and the dew point temperature versus the % air. Comment. 2) For 120% dry-air at 1 atm, 25 C, tabulate and plot the heat transfer in KJ per kmole of natural gas versus product temperature. Vary the product temperature from 25 C (assume water in vapor form for 25 C point for heat transfer calculations) to 2000 K. Consider 298 K, 500 K, 1000 K, 1500 K, 2000 K points. Comment. You are to develop an interactive computer program (Matlab or any computer programming code) on fuel combustion. Your program should balance the combustioin equation of any hydrocarbon fuel (CDH,) for 100% or higher amount of air. Assume dry air available at 25 C, 1 atm. Assume complete combustion, therefore, the products carn include CO2, H20, O2, and N2. Your developed program output should include: (work in SI units by yourself or groups of two students) Complete combustion equation when fuel type and percent dry-air are given (total products pressure is 1 atm). atm) and temperature are given. *Air fuel ratio on a mass basis and on a mole basis, water dew point temperature Heat transfer calculations when reactants (1 atm, 25 C) and products pressure (1 a) Run your source code for natural gas available as 80% methane (CH4) and 20% ethane (C2Hs) on a molar basis at 25 C to develop the following: You need to show complete showing sample formulations/calculations for at your ammi ar your piogram 1) For a range of % dry-air from 100% to 200% (increments of 20%) tabulate and plot: AF ratio (mass/mole basis), and the dew point temperature versus the % air. Comment. 2) For 120% dry-air at 1 atm, 25 C, tabulate and plot the heat transfer in KJ per kmole of natural gas versus product temperature. Vary the product temperature from 25 C (assume water in vapor form for 25 C point for heat transfer calculations) to 2000 K. Consider 298 K, 500 K, 1000 K, 1500 K, 2000 K points. Comment
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


