Question: Max collected the following data for Part 2. Refer to this data in answering Questions 11 through 13. Glassware used to Measure 10 mL Aliquots:
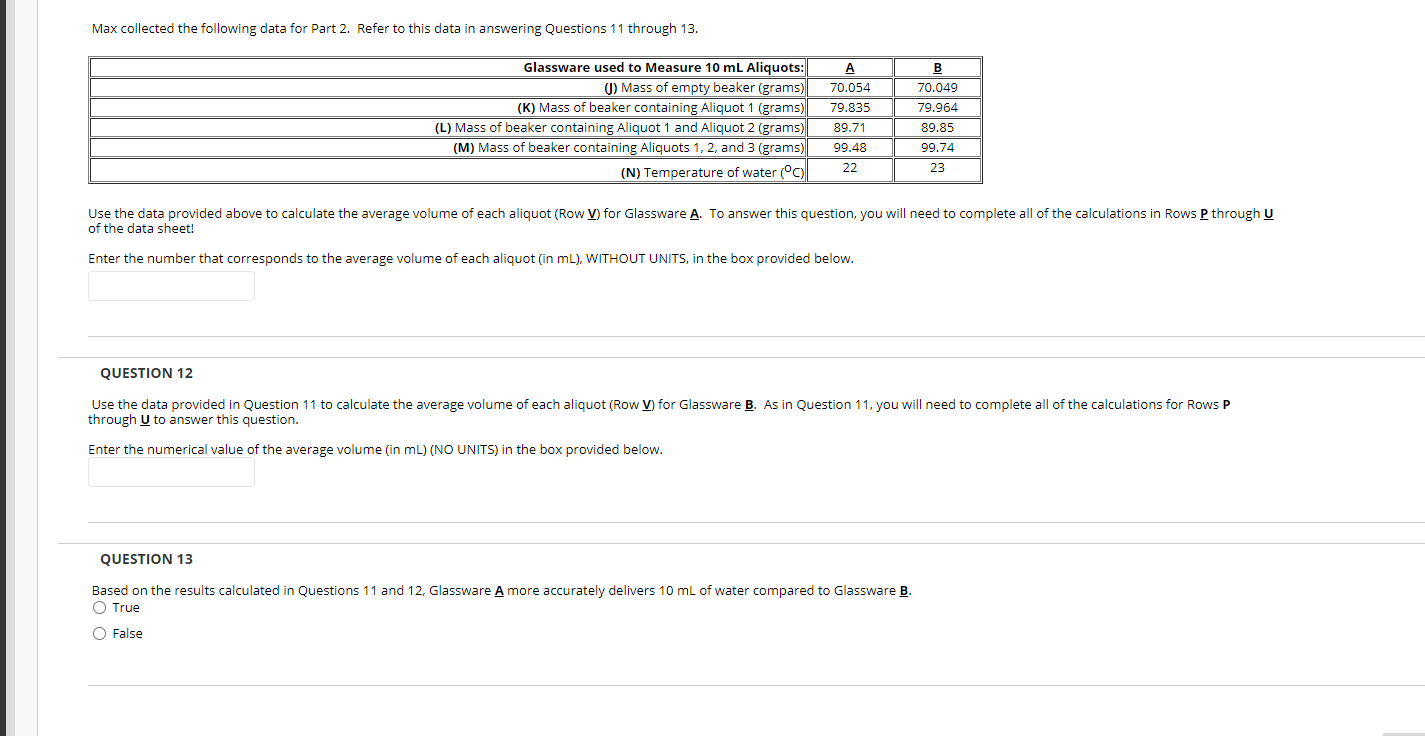
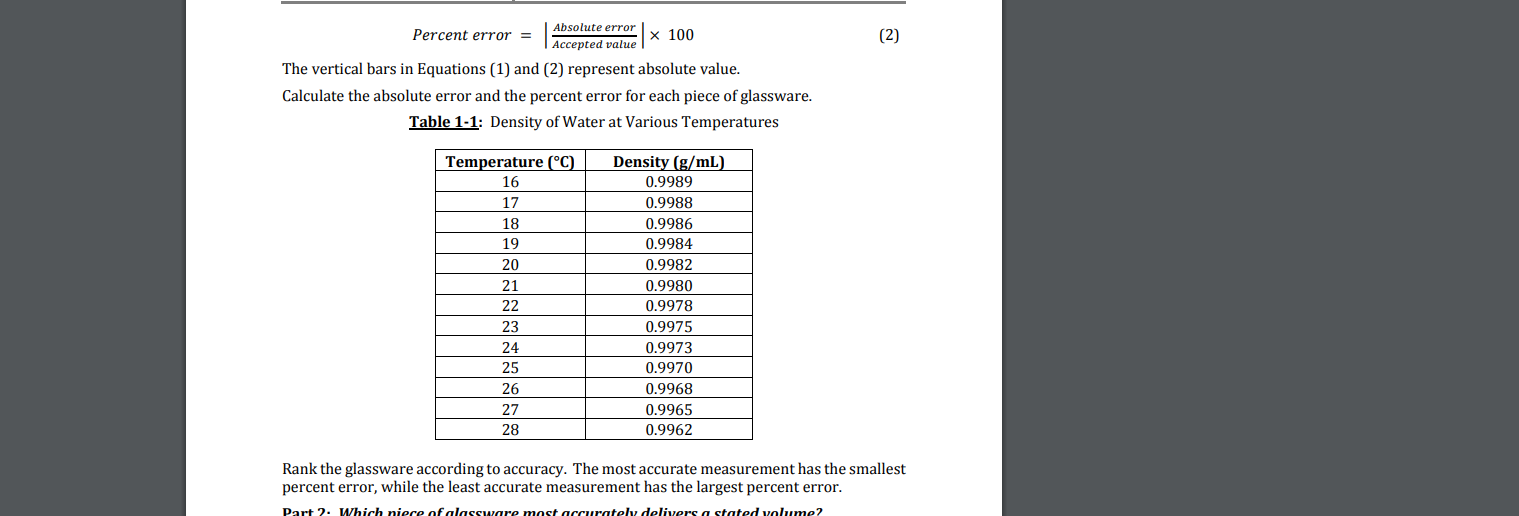
Max collected the following data for Part 2. Refer to this data in answering Questions 11 through 13. Glassware used to Measure 10 mL Aliquots: (1) Mass of empty beaker (grams) (K) Mass of beaker containing Aliquot 1 (grams) (L) Mass of beaker containing Aliquot 1 and Aliquot 2 (grams) (M) Mass of beaker containing Aliquots 1, 2, and 3 (grams) (N) Temperature of water () A 70.054 79.835 89.71 99.48 22 B 70.049 79.964 89.85 99.74 23 Use the data provided above to calculate the average volume of each aliquot (Row V) for Glassware A. To answer this question, you will need to complete all of the calculations in Rows P through U of the data sheet! Enter the number that corresponds to the average volume of each aliquot (in mL), WITHOUT UNITS, in the box provided below. QUESTION 12 Use the data provided in Question 11 to calculate the average volume of each aliquot (Row V) for Glassware B. As in Question 11, you will need to complete all of the calculations for Rows P through U to answer this question. Enter the numerical value of the average volume (in mL) (NO UNITS) in the box provided below. QUESTION 13 Based on the results calculated in Questions 11 and 12, Glassware A more accurately delivers 10 mL of water compared to Glassware B. O True O False (2) Absolute error Percent error = X 100 Accepted value The vertical bars in Equations (1) and (2) represent absolute value. Calculate the absolute error and the percent error for each piece of glassware. Table 1-1: Density of Water at Various Temperatures Temperature (C) 16 17 18 19 20 21 22 23 24 25 26 27 28 Density (g/mL) 0.9989 0.9988 0.9986 0.9984 0.9982 0.9980 0.9978 0.9975 0.9973 0.9970 0.9968 0.9965 0.9962 Rank the glassware according to accuracy. The most accurate measurement has the smallest percent error, while the least accurate measurement has the largest percent error. Part 2. Which niece of alassware most accurately delivers a stated volume? Max collected the following data for Part 2. Refer to this data in answering Questions 11 through 13. Glassware used to Measure 10 mL Aliquots: (1) Mass of empty beaker (grams) (K) Mass of beaker containing Aliquot 1 (grams) (L) Mass of beaker containing Aliquot 1 and Aliquot 2 (grams) (M) Mass of beaker containing Aliquots 1, 2, and 3 (grams) (N) Temperature of water () A 70.054 79.835 89.71 99.48 22 B 70.049 79.964 89.85 99.74 23 Use the data provided above to calculate the average volume of each aliquot (Row V) for Glassware A. To answer this question, you will need to complete all of the calculations in Rows P through U of the data sheet! Enter the number that corresponds to the average volume of each aliquot (in mL), WITHOUT UNITS, in the box provided below. QUESTION 12 Use the data provided in Question 11 to calculate the average volume of each aliquot (Row V) for Glassware B. As in Question 11, you will need to complete all of the calculations for Rows P through U to answer this question. Enter the numerical value of the average volume (in mL) (NO UNITS) in the box provided below. QUESTION 13 Based on the results calculated in Questions 11 and 12, Glassware A more accurately delivers 10 mL of water compared to Glassware B. O True O False (2) Absolute error Percent error = X 100 Accepted value The vertical bars in Equations (1) and (2) represent absolute value. Calculate the absolute error and the percent error for each piece of glassware. Table 1-1: Density of Water at Various Temperatures Temperature (C) 16 17 18 19 20 21 22 23 24 25 26 27 28 Density (g/mL) 0.9989 0.9988 0.9986 0.9984 0.9982 0.9980 0.9978 0.9975 0.9973 0.9970 0.9968 0.9965 0.9962 Rank the glassware according to accuracy. The most accurate measurement has the smallest percent error, while the least accurate measurement has the largest percent error. Part 2. Which niece of alassware most accurately delivers a stated volume
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


