Question: Maxwell-Boltzmann energy distribution: n(E)=NCexp(KTE) Problem: The two lines on the graph are the same gas (both type and amount) for two different temperature. Number of
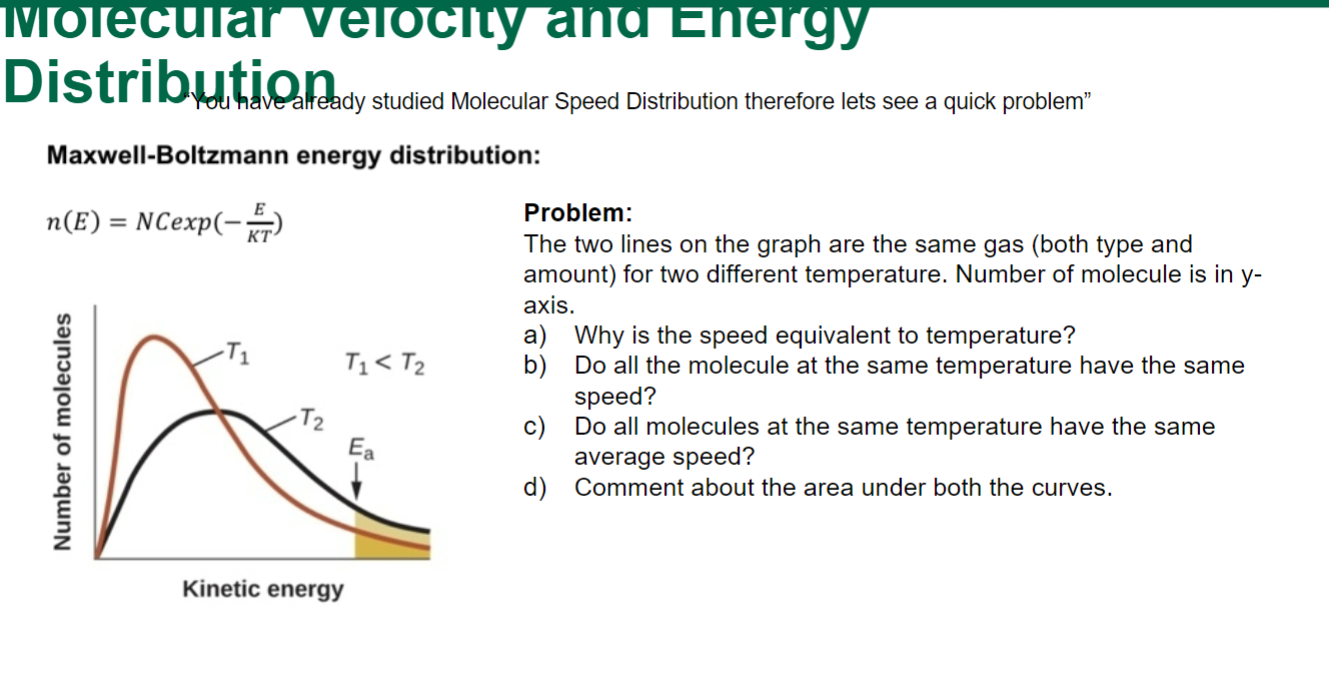
Maxwell-Boltzmann energy distribution: n(E)=NCexp(KTE) Problem: The two lines on the graph are the same gas (both type and amount) for two different temperature. Number of molecule is in yaxis. a) Why is the speed equivalent to temperature? b) Do all the molecule at the same temperature have the same speed? c) Do all molecules at the same temperature have the same average speed? d) Comment about the area under both the curves
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


