Question: may you please answer both The equilibrium constant is 0.0900 at 25C for the following reaction: H2O(g) + Cl2O(g) =2HOCI(9) If 2.1 atm of H20
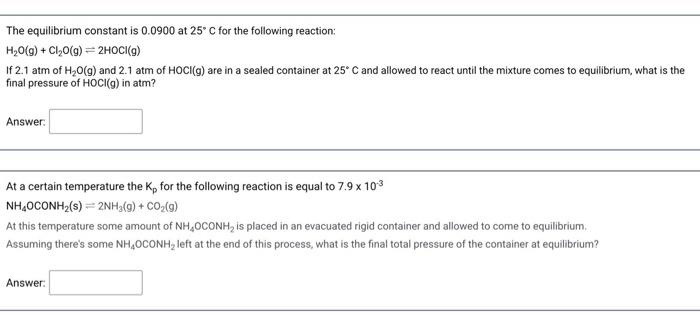
The equilibrium constant is 0.0900 at 25C for the following reaction: H2O(g) + Cl2O(g) =2HOCI(9) If 2.1 atm of H20 (9) and 2.1 atm of HOCI(g) are in a sealed container at 25C and allowed to react until the mixture comes to equilibrium, what is the final pressure of HOCI(g) in atm? Answer: At a certain temperature the K, for the following reaction is equal to 7.9 x 103 NH,OCONH2(s) =2NH3(g) + CO2(9) At this temperature some amount of NH,OCONH, is placed in an evacuated rigid container and allowed to come to equilibrium Assuming there's some NHOCONH, left at the end of this process, what is the final total pressure of the container at equilibrium
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


