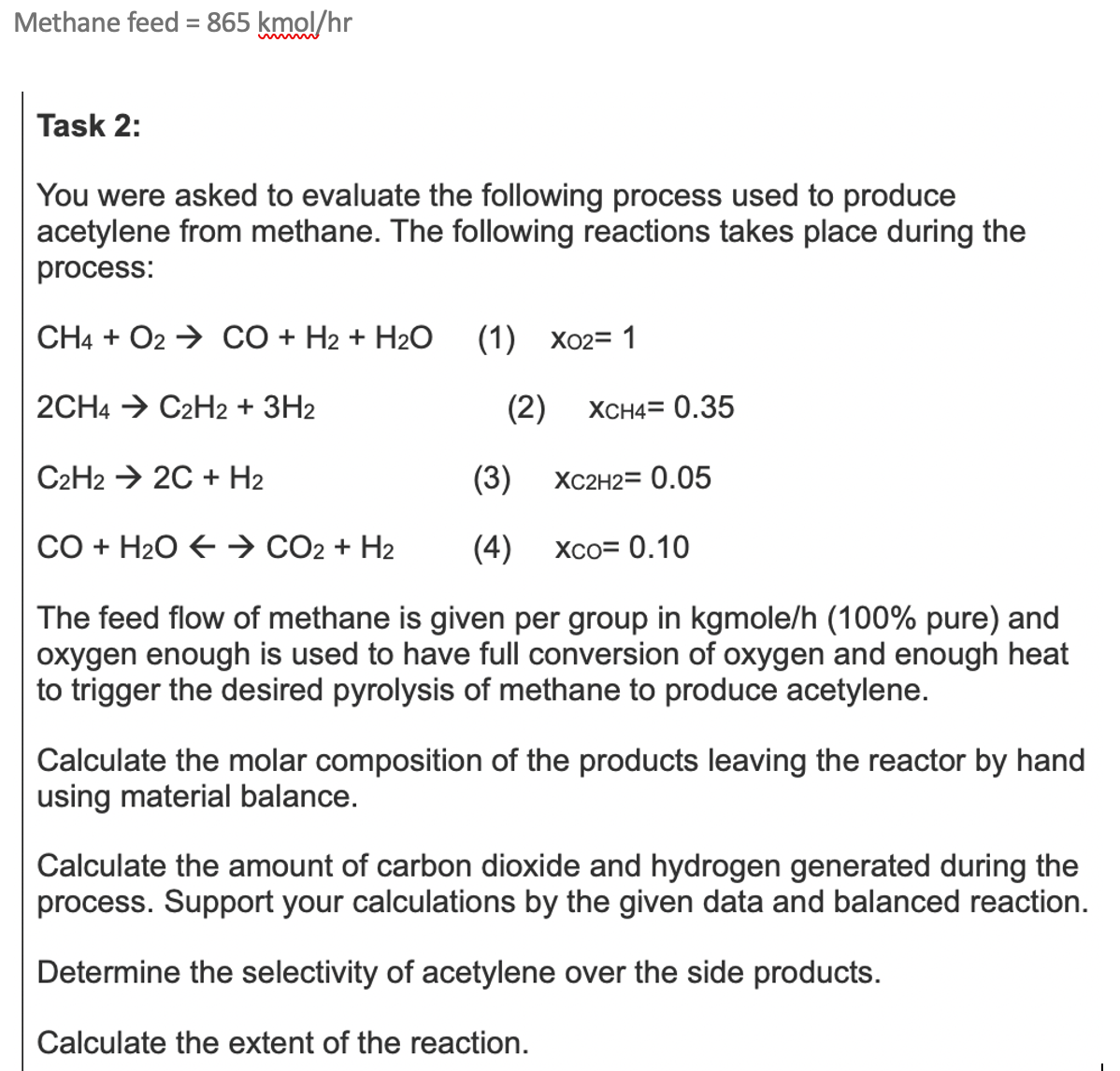
Question: Methane feed = 8 6 5 kmo l h r Task 2 : You were asked to evaluate the following process used to produce acetylene
Methane feed kmo
Task :
You were asked to evaluate the following process used to produce
acetylene from methane. The following reactions takes place during the
process:
Olarr
The feed flow of methane is given per group in kgmoleh pure and
oxygen enough is used to have full conversion of oxygen and enough heat
to trigger the desired pyrolysis of methane to produce acetylene.
Calculate the molar composition of the products leaving the reactor by hand
using material balance.
Calculate the amount of carbon dioxide and hydrogen generated during the
process. Support your calculations by the given data and balanced reaction.
Determine the selectivity of acetylene over the side products.
Calculate the extent of the reaction.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


