Question: Methanol and ethanol solutions are basically ideal. a ) Calculate the vapor - liquid equilibria for this system at 1 and 5 atm absolute pressure
Methanol and ethanol solutions are basically ideal.
a Calculate the vaporliquid equilibria for this system at and atm absolute pressure and plot the xy and txy diagrams at each pressure.
b For each pressure, calculate the relative volatilities and determine an average value.
c Using equation with the average volatilities, compare the values of ya at each value of x obtained in this way with the values calculated directly from the vapor pressures.
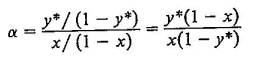
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


