Question: Methanol is a liquid at room temperature with a boiling point of 64.7C. It can be synthesized from a mixture of carbon monoxide and hydrogen
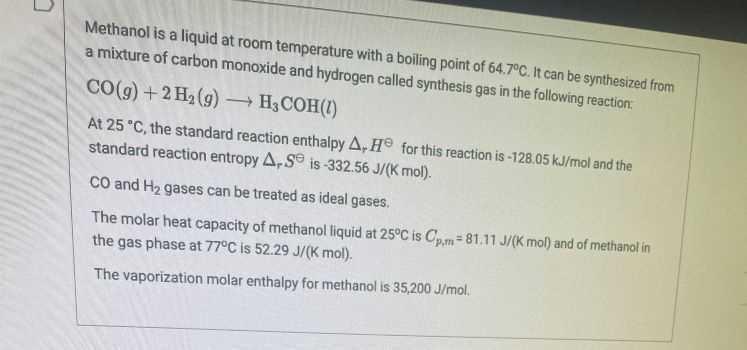
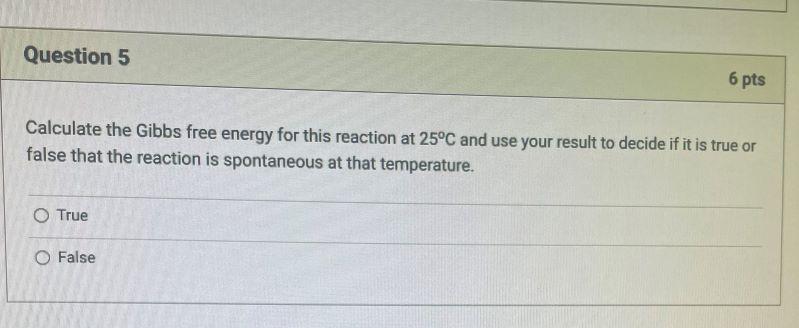
Methanol is a liquid at room temperature with a boiling point of 64.7C. It can be synthesized from a mixture of carbon monoxide and hydrogen called synthesis gas in the following reaction, CO(g) + 2 H2(g) H3COH(1) At 25 C, the standard reaction enthalpy A, H for this reaction is -128.05 kJ/mol and the standard reaction entropy A-S is -332.56 J/(k mol). CO and Hz gases can be treated as ideal gases The molar heat capacity of methanol liquid at 25C is Opm=81.11 J/(k mol) and of methanol in the gas phase at 77C is 52.29 J/(K mol). The vaporization molar enthalpy for methanol is 35,200 J/mol. Question 5 6 pts Calculate the Gibbs free energy for this reaction at 25C and use your result to decide if it is true or false that the reaction is spontaneous at that temperature. O True O False
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


