Question: Methanol is a liquid at room temperature with a boiling point of 64.7C. It can be synthesized from a mixture of carbon monoxide and hydrogen
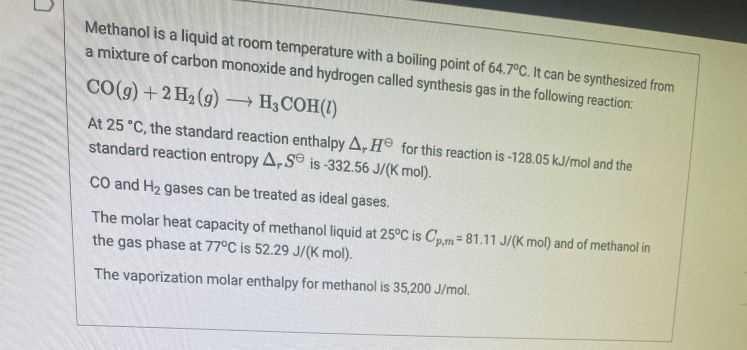
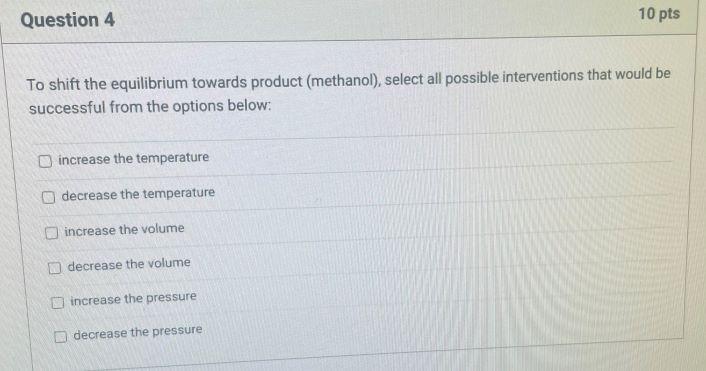
Methanol is a liquid at room temperature with a boiling point of 64.7C. It can be synthesized from a mixture of carbon monoxide and hydrogen called synthesis gas in the following reaction, CO(g) + 2 H2(g) H3COH(1) At 25 C, the standard reaction enthalpy A, H for this reaction is -128.05 kJ/mol and the standard reaction entropy A-S is -332.56 J/(k mol). CO and Hz gases can be treated as ideal gases The molar heat capacity of methanol liquid at 25C is Opm=81.11 J/(k mol) and of methanol in the gas phase at 77C is 52.29 J/(K mol). The vaporization molar enthalpy for methanol is 35,200 J/mol. Question 4 10 pts To shift the equilibrium towards product (methanol), select all possible interventions that would be successful from the options below: increase the temperature decrease the temperature increase the volume decrease the volume increase the pressure decrease the pressure
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


