Question: MISSED THIS? Read Section 14.7 (Pages 613 - 616 ) . Using the van't Holf factors in the table below, calculate the mass of solute
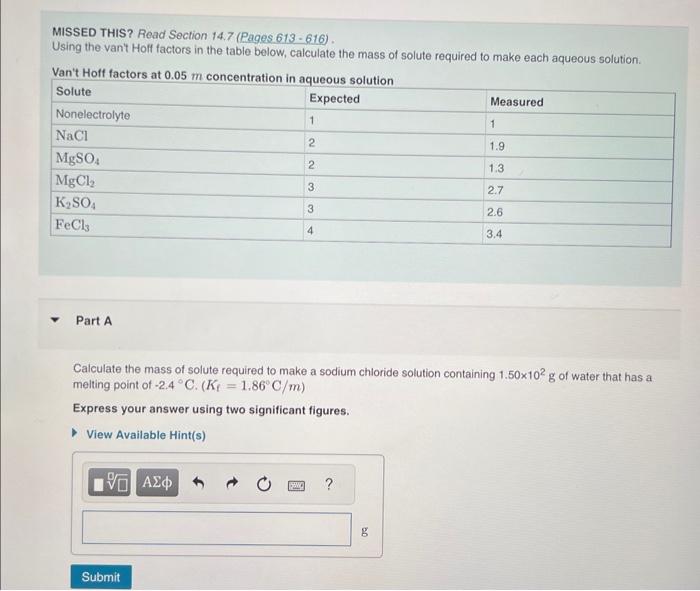
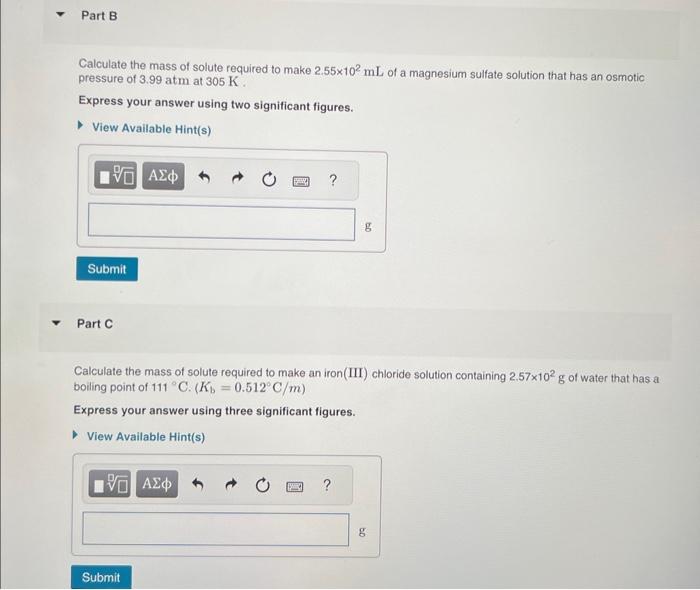
MISSED THIS? Read Section 14.7 (Pages 613 - 616 ) . Using the van't Holf factors in the table below, calculate the mass of solute required to make each aqueous solution. Van't Hoff factare at n ne Part A Calculate the mass of solute required to make a sodium chioride solution containing 1.50102g of water that has a melting point of 2.4C(Kf=1.86C/m) Express your answer using two significant figures. Calculate the mass of solute required to make 2.55102mL of a magnesium sulfate solution that has an osmotic pressure of 3.99atm at 305K. Express your answer using two significant figures. Part C Calculate the mass of solute required to make an iron(III) chloride solution containing 2.57102g of water that has a boiling point of 111C.(Kb=0.512C/m) Express your answer using three significant figures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


