Question: MISSED THIS? Read Section 14.7 (Pages 613 - 616). Using the van't Hotf factors in the table below, calculate the mass of solute required to

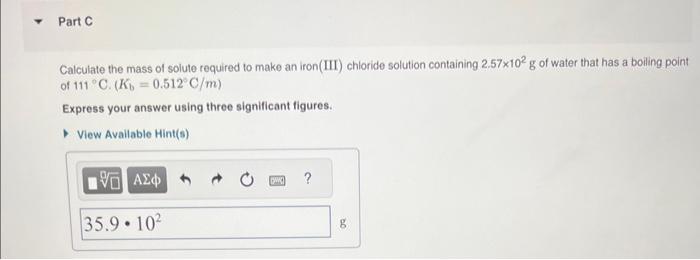
MISSED THIS? Read Section 14.7 (Pages 613 - 616). Using the van't Hotf factors in the table below, calculate the mass of solute required to make each aqueous solution. Calculate the mass of solute required to make an iron(III) chloride solution containing 2.57102g of water that has a boiling point of 111C.(Kb=0.512C/m) Express your answer using three significant figures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


