Question: MISSED THIS? Read Section 15.3 (Pages 637 - 642) . The following reaction is first order in N2O5 : N2O5(g)NO3(g)+NO2(g) The rate constant for the

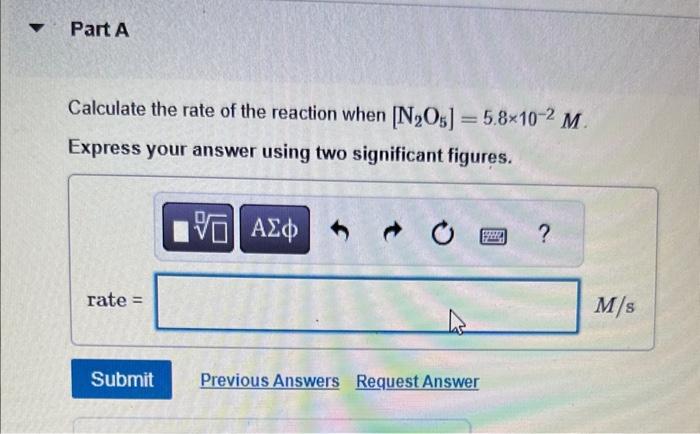
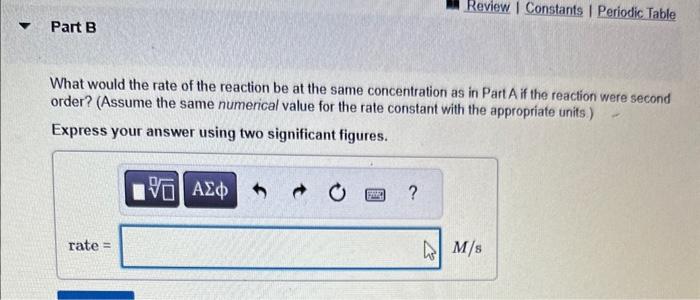
MISSED THIS? Read Section 15.3 (Pages 637 - 642) . The following reaction is first order in N2O5 : N2O5(g)NO3(g)+NO2(g) The rate constant for the reaction at a certain temperature is 0.053/s. Calculate the rate of the reaction when [N2O5]=5.8102M. Express your answer using two significant figures. What would the rate of the reaction be at the same concentration as in Part A if the reaction were second order? (Assume the same numerical value for the rate constant with the appropriate units ) Express your answer using two significant figures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


