Question: MISSED THIS? Read Section 15.6 ; Watch KCV 15.6, IWE 15.9. What is the overall reaction? Consider the following two-step mechanism for a reaction: Express
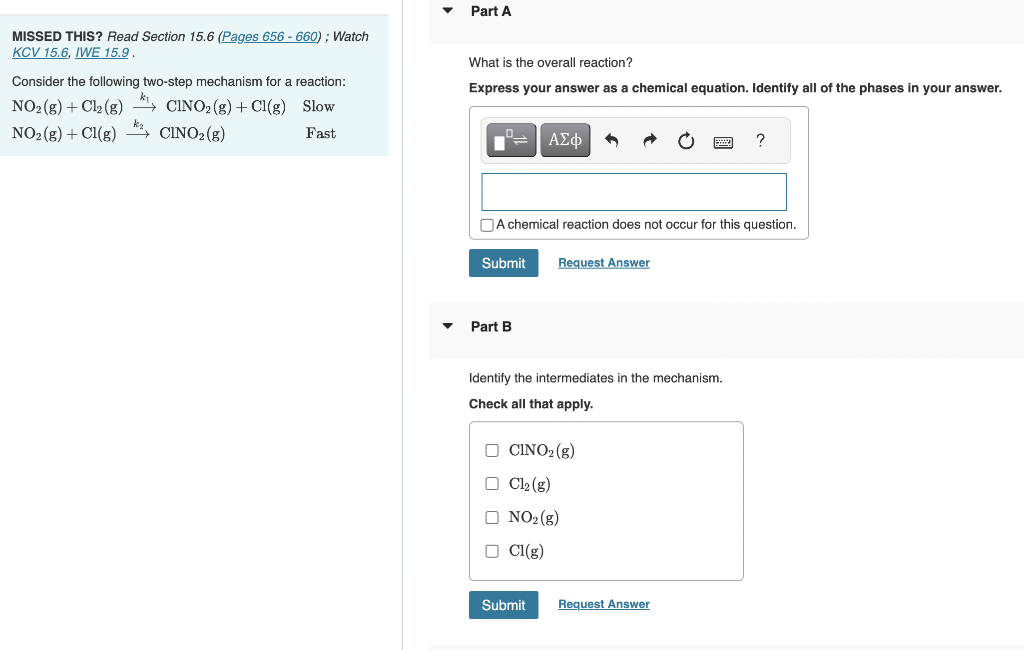
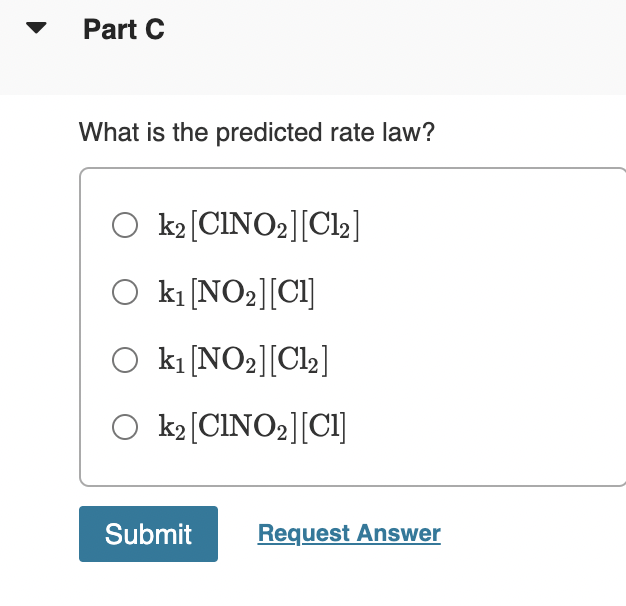
MISSED THIS? Read Section 15.6 ; Watch KCV 15.6, IWE 15.9. What is the overall reaction? Consider the following two-step mechanism for a reaction: Express your answer as a chemical equation. Identify all of the phases in your answer. NO2(g)+Cl2(g)k1ClNO2(g)+Cl(g)SlowNO2(g)+Cl(g)k2ClNO2(g)FastAchemicalreactiondoesnotoccurforthisquestion. Part B Identify the intermediates in the mechanism. Check all that apply. ClNO2(g)Cl2(g)NO2(g)Cl(g) What is the predicted rate law? k2[ClNO2][Cl2]k1[NO2][Cl]k1[NO2][Cl2]k2[ClNO2][Cl]
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


