Question: MISSED THIS? Read Section 16.6 (Pages 696 - 699); Watch WWE 16.5 Calculate the equilibrium constant (Kc) for the reaction at this temperature. Consider the
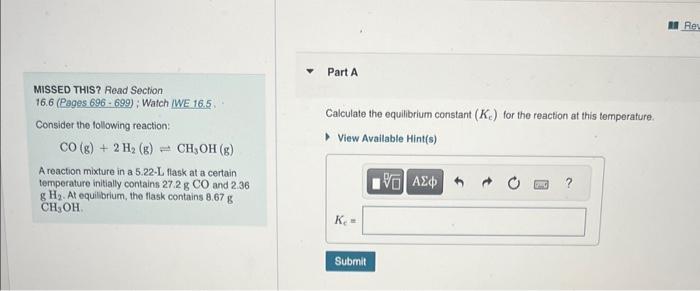
MISSED THIS? Read Section 16.6 (Pages 696 - 699); Watch WWE 16.5 Calculate the equilibrium constant (Kc) for the reaction at this temperature. Consider the following reaction: CO(g)+2H2(g)CH3OH(g) A reaction mixture in a 5.22-L flask at a certain temperature initially contains 27.2gCO and 2.36 gH2. At equilbrium, the flask contains 8.67g CH3OH
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


