Question: please explain how to set up ice table and how to find kc Caiculate the value of the equilibrium constant (Kc). MISSED THIS? Read Section
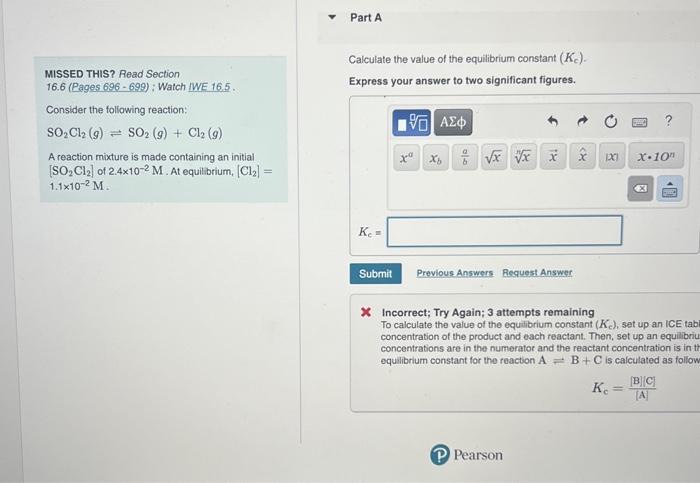
Caiculate the value of the equilibrium constant (Kc). MISSED THIS? Read Section 16.6 (Pages 696 - 699) ; Watch IWE 16.5. Express your answer to two significant figures. Consider the following reaction: SO2Cl2(g)SO2(g)+Cl2(g) A reaction mixture is made containing an initial [SO2Cl2] of 2.4102M. At equilibrium, [Cl2]= 1.1102M. x Incorrect; Try Again; 3 attempts remaining To calculate the value of the equilibrium constant (Kc), set up an ICE tabi concentration of the product and each reactant. Then, set up an equilibriu concentrations are in the numerator and the reactant concentration is in tt equilibrium constant for the reaction AB+C is calculated as follow Kc=[A][B][C]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


