Question: MISSED THIS? Read Section 16.8 (Pages 701 - 710) ; Watch KCV 16.8, IWE 16.12. Consider the following reaction: f a solution initially contains 0.170MHC2H3O2,
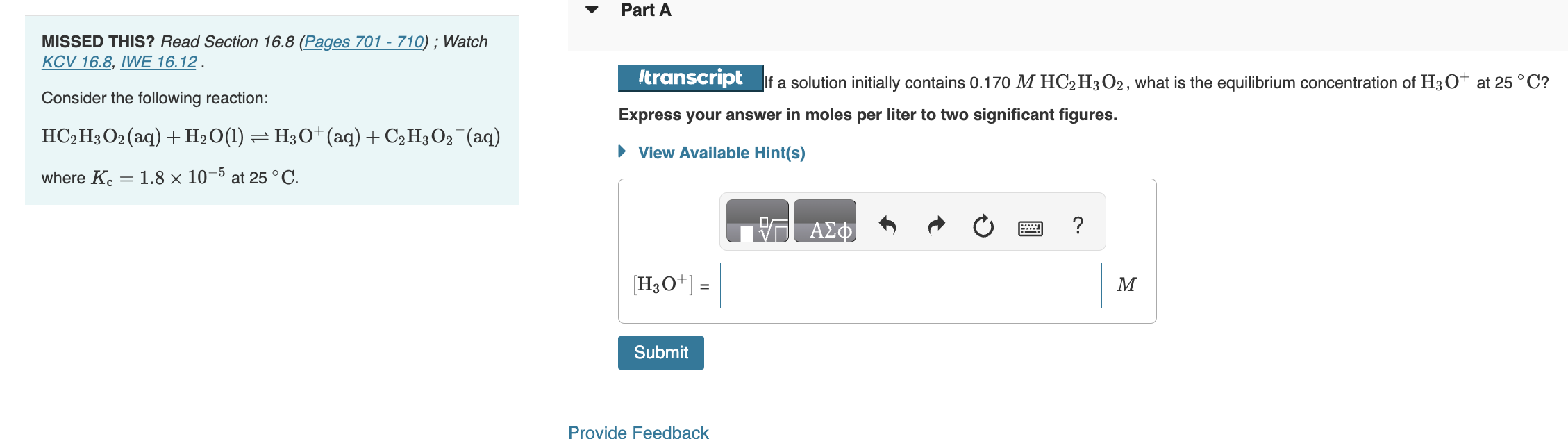
MISSED THIS? Read Section 16.8 (Pages 701 - 710) ; Watch KCV 16.8, IWE 16.12. Consider the following reaction: f a solution initially contains 0.170MHC2H3O2, what is the equilibrium concentration of H3O+at 25C ? HC2H3O2(aq)+H2O(l)H3O+(aq)+C2H3O2(aq) Express your answer in moles per liter to two significant figures. where Kc=1.8105 at 25C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


