Question: MISSED THIS? Read Section 16.8 (Pages 701 - 710) ; Watch KCV 16.8. LWE 16.9. Consider the reaction NiO(s)+CO(g)Ni(s)+CO2(g) for which Ke=4.0103 at 1500K. Part
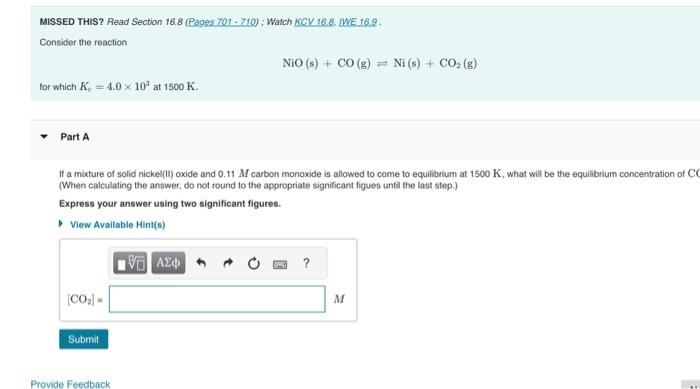
MISSED THIS? Read Section 16.8 (Pages 701 - 710) ; Watch KCV 16.8. LWE 16.9. Consider the reaction NiO(s)+CO(g)Ni(s)+CO2(g) for which Ke=4.0103 at 1500K. Part A If a mixture of solid nickel(1i) oxide and 0.11M carbon monoxide is allowed to come to equilibrum at 1500K, what will be the equilibrium concentration of C (When calculating the answer, do not round to the appropriate significant figues untit the last step.) Express your answer using two significant figures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


