Question: MISSED THIS? Read Section 6.6 (Rages 228 - 285 ) ; Warch KCV 6.6 A gas mixture with a total pressure of 770mmHg contains each
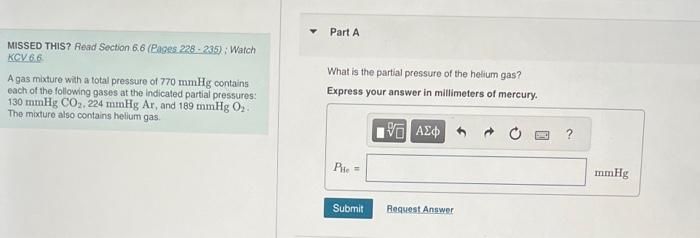
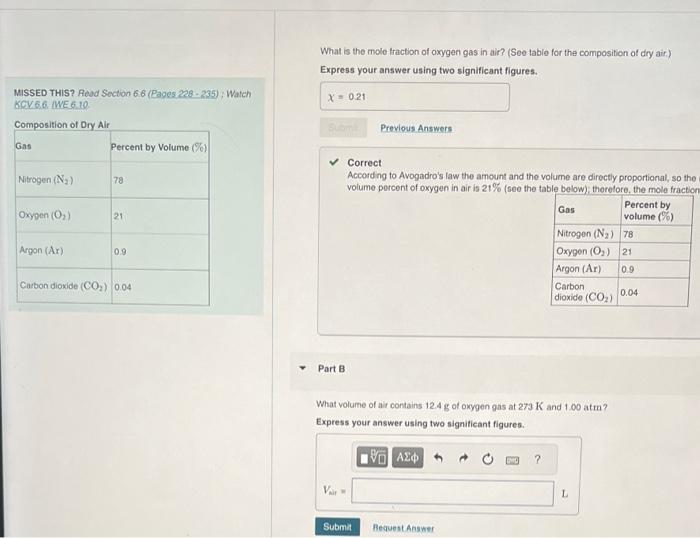
MISSED THIS? Read Section 6.6 (Rages 228 - 285 ) ; Warch KCV 6.6 A gas mixture with a total pressure of 770mmHg contains each of the following gases at the indicated partial pressures: What is the partial pressure of the helium gas? 130mmHgCO2,224mmHgAr, and 189mmHg2. The mixture also contains hellum gas. What is the mole traction of oxygen gas in air? (See table for the composition of dry air) Express your answer using two significant figures. MisSED THIS? Reod Section 6.6 (Pages 228 - 235) ; Watch KCY 6.6. MVE 6.10 Correct According to Avogadro's law the amount and the volume are directly proportional, so the volume percent of oxygen in air is 21% (seo the table below): thorelore, the mole fraction Part B What volume of air contains 12.4g of oxygen gas at 273K and 1.00 atm? Express your answer using two signiticant figures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


