Question: Id we connect the two flasks through a stopcrock and we open the stopcrock, what is the partial pressure of helium? Express to three significant
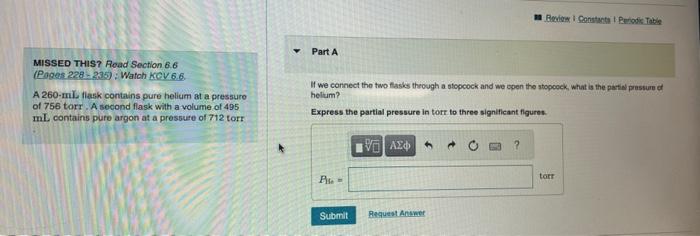
MISSED THIS? Read Section 6.6 (Pages 228-235): Watch Mrey 6.6. If we connect the two fasks theough a olopcock and we epen the atopoock, what is the part pressure of A 260-mil liak contains pure helium at a pressure heltum? of 756 tory. A second flask with a volume of 495 mL contains pure argon at a pressure of 712 tort Express the partial pressure in torf to three signilicant flgures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


