Question: MISSED THIS? Watch KCV: Colligative Properties, IWE: Boiling Point Elevation; Read Section 14.6. You can click on the Review link to access the section in
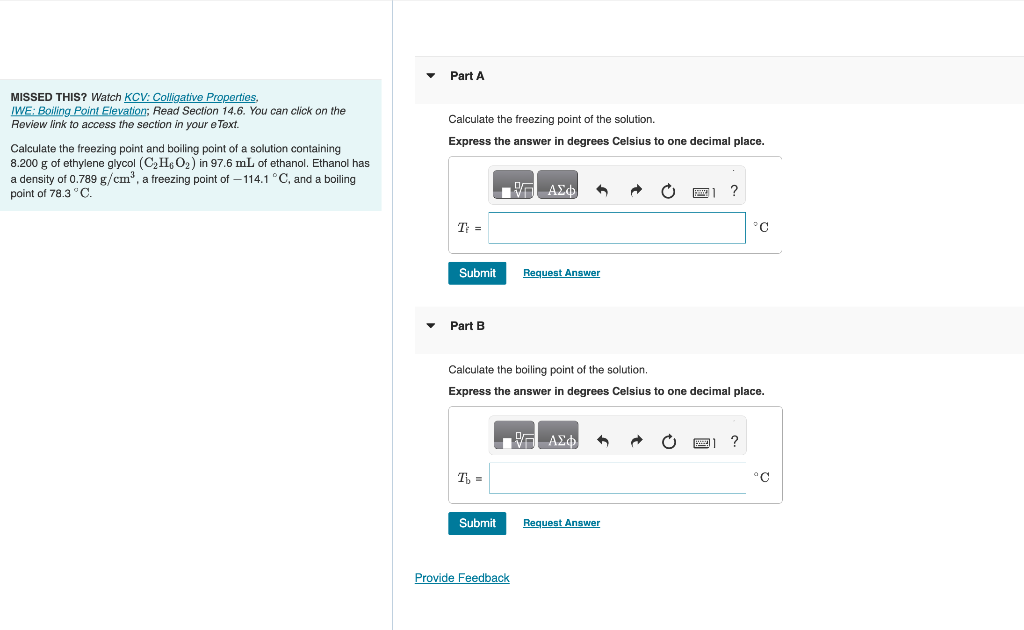
MISSED THIS? Watch KCV: Colligative Properties, IWE: Boiling Point Elevation; Read Section 14.6. You can click on the Review link to access the section in your e Text. Calculate the freezing point of the solution. Calculate the freezing point and boiling point of a solution containing Express the answer in degrees Celsius to one decimal place. 8.200g of ethylene glycol (C2H6O2) in 97.6mL of ethanol. Ethanol has a density of 0.789g/cm3, a freezing point of 114.1C, and a boiling point of 78.3C. Part B Calculate the boiling point of the solution. Express the answer in degrees Celsius to one decimal place
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


