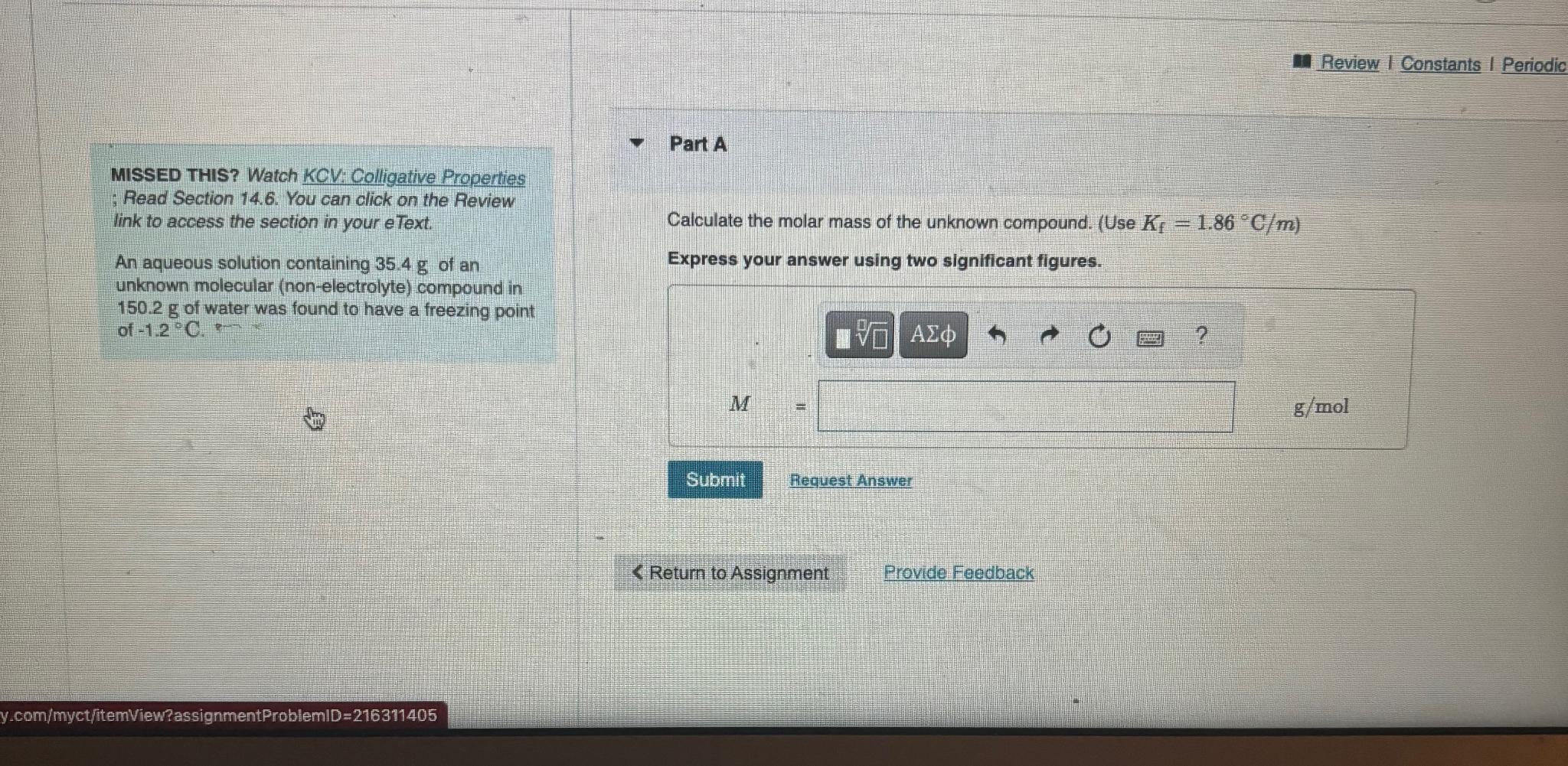
Question: MISSED THIS? Watch KCV: Colligative Properties ; Read Section 1 4 . 6 . You can click on the Review link to access the section
MISSED THIS? Watch KCV: Colligative Properties ; Read Section You can click on the Review link to access the section in your eText.
An aqueous solution containing of an unknown molecular nonelectrolyte compound in of water was found to have a freezing point of
Part A
Calculate the molar mass of the unknown compound. Use
Express your answer using two significant figures.
Return to Assignment
Provide Feedback
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


