Question: MISSED THIS? Watch KCV: Limiting Reactant, Theoretical Yield, and 5molMn and 5molO2 Percent Yield Express your answer using one significant figure. IVE: Limiting Reactant and
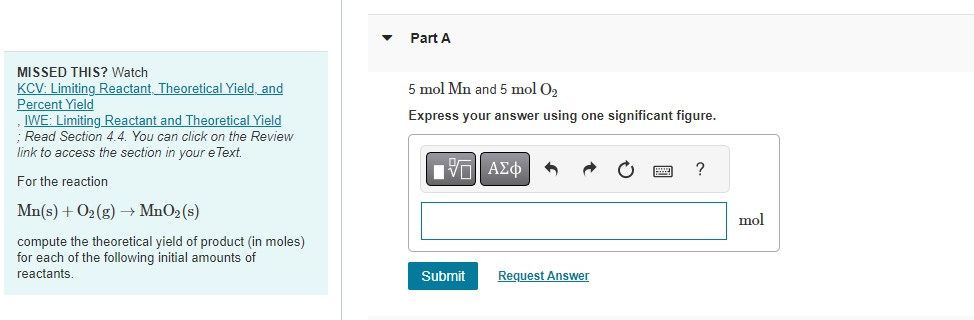
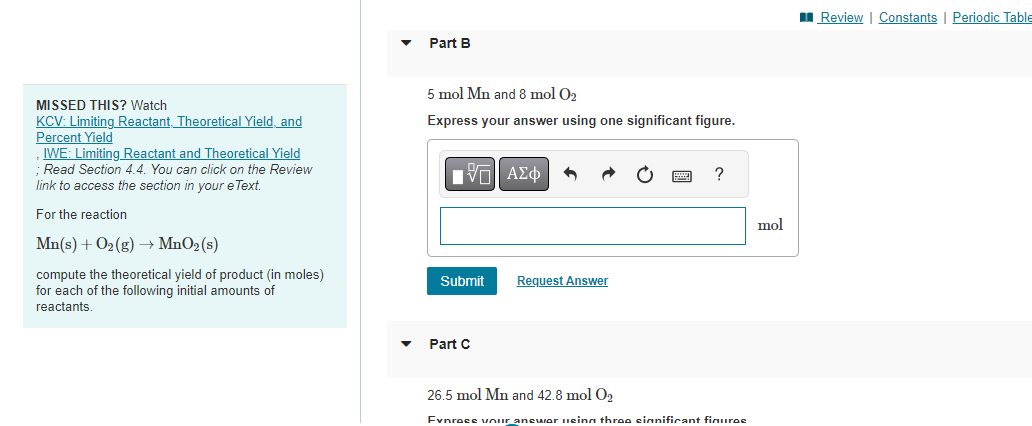
MISSED THIS? Watch KCV: Limiting Reactant, Theoretical Yield, and 5molMn and 5molO2 Percent Yield Express your answer using one significant figure. IVE: Limiting Reactant and Theoretical Yield ; Read Section 4.4. You can click on the Review link to access the section in your eText. For the reaction Mn(s)+O2(g)MnO2(s) compute the theoretical yield of product (in moles) for each of the following initial amounts of reactants. MISSED THIS? Watch 5molMn and 8molO2 KCV: Limiting Reactant, Theoretical Yield, and Express your answer using one significant figure. Percent Yield IWE: Limiting Reactant and Theoretical Yield ; Read Section 4.4. You can click on the Review link to access the section in your eText. For the reaction Mn(s)+O2(g)MnO2(s) compute the theoretical yield of product (in moles) for each of the following initial amounts of reactants. Part C 26.5molMn and 42.8molO2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


