Question: MISSED THIS? Watch KCV: Limiting Reactant, Theoretical Yield, and Percent Yield, IWE: Limiting Reactant and Theoretical Yield; Read Section 4.4. You can click on the

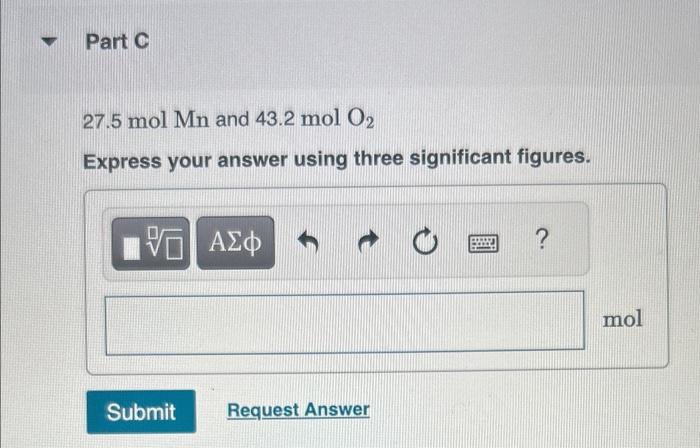

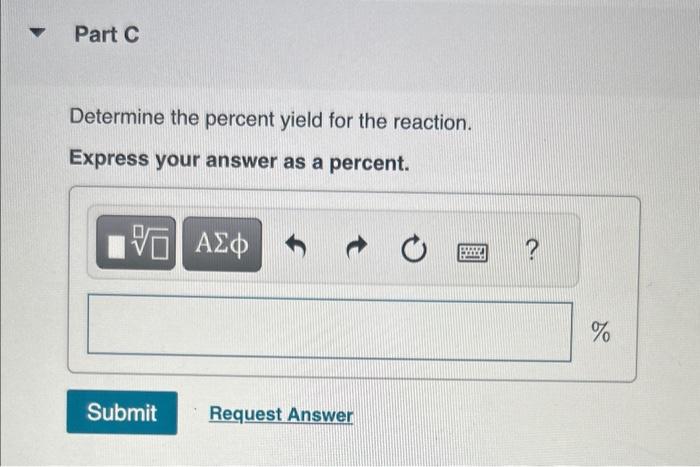
MISSED THIS? Watch KCV: Limiting Reactant, Theoretical Yield, and Percent Yield, IWE: Limiting Reactant and Theoretical Yield; Read Section 4.4. You can click on the Review link to access the section in your eText. For the reaction Mn(s)+O2(g)MnO2(s) compute the theoretical yield of product (in moles) for each of the following initial amounts of reactants. 27.5molMn and 43.2molO2 Express your answer using three significant figures. MISSED THIS? Watch KCV: Limiting Reactant, Theoretical Yield, and Percent Yield, IWE: Limiting Reactant and Theoretical Yield; Read Section 4.4. You can click on the Review link to access the section in your eText. Magnesium oxide can be made by heating magnesium metal in the presence of the oxygen. The balanced equation for the reaction is: 2Mg(s)+O2(g)2MgO(s) When 13.2gMg reacts with 14.0gO2,12.5gMgO is collected. Determine the percent yield for the reaction. Express your answer as a percent
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


