Question: mocentrabion f (4pts) Complete the missing information in this formulation to prepare 1 fluid ounce of 10% w/v hydrochloric acid solution. Recall that concentrated acids
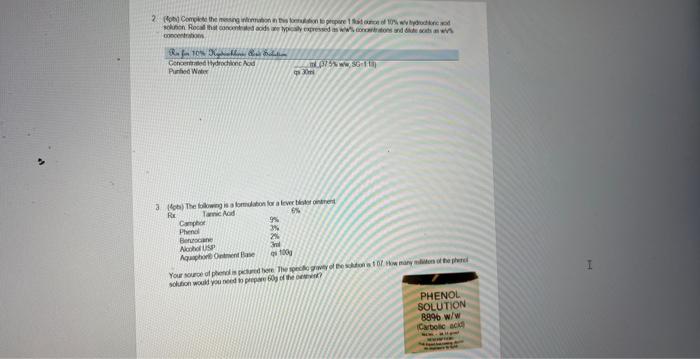



mocentrabion f (4pts) Complete the missing information in this formulation to prepare 1 fluid ounce of 10% w/v hydrochloric acid solution. Recall that concentrated acids are typically expressed as w/w\% concentrations and dilute acids as w/v\% concentrations. 3. (4pts) The following is a formulation for a fever blister ointment. Rx Thmesahn=1 Your source of phenol is pictured here. The specific gravity of the solution is 1.07. How many milliters af the phenol solution would you need to prepare 60g of the ointment? (4pts) The following is a formulation for a fever blister ointment. Your source of phenol is pictured here. The specific gravity of the solution is 1.07. How many millititers of the phenol solution would you need to prepare 60g of the ointment? mocentrabion f (4pts) Complete the missing information in this formulation to prepare 1 fluid ounce of 10% w/v hydrochloric acid solution. Recall that concentrated acids are typically expressed as w/w\% concentrations and dilute acids as w/v\% concentrations. 3. (4pts) The following is a formulation for a fever blister ointment. Rx Thmesahn=1 Your source of phenol is pictured here. The specific gravity of the solution is 1.07. How many milliters af the phenol solution would you need to prepare 60g of the ointment? (4pts) The following is a formulation for a fever blister ointment. Your source of phenol is pictured here. The specific gravity of the solution is 1.07. How many millititers of the phenol solution would you need to prepare 60g of the ointment
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


