Question: Model 1: Common charges (by group) on elements when in lonic compounde. A cation nas a positive cnarge. Critical Thinking Questions: 1. Recall the shell
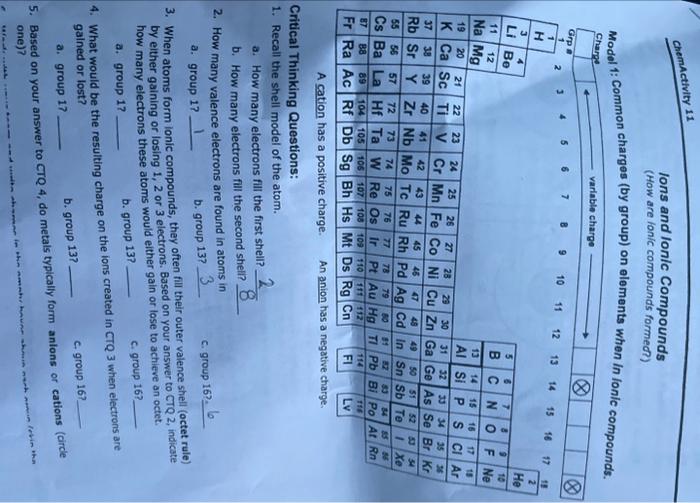
Model 1: Common charges (by group) on elements when in lonic compounde. A cation nas a positive cnarge. Critical Thinking Questions: 1. Recall the shell model of the atom. a. How many electrons fill the first shell? 82 b. How many electrons fill the second sheli? 2. How many valence electrons are found in atoms in a. group 1? b. group 13? c. group 16?, be 3. When atoms form ionic compounds, they often fill their outer valence shell (octet rule) by elther 9 aining or losing 1, 2 or 3 electrons. Based on your answer to CTQ 2, indicate how many electrons these atoms would either gain or lose to achieve an octet. a. group 1 ? b. group 13? C. group 16? 4. What would be the resulting charge on the ions created in CTQ3 when electrons are gained or lost? a. group 17 b. group 13? c. group 16? 5. Based on your answer to CTQ 4 , do metals typically form anions or cations (circle one)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


